H0920000
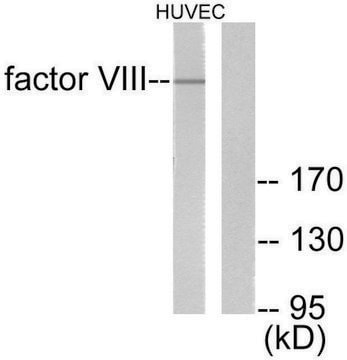
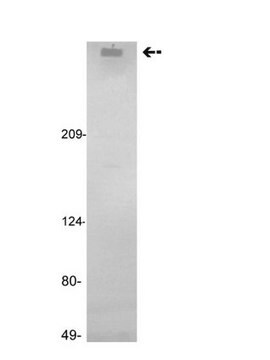
Human coagulation factor VIII concentrate
European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
grade
pharmaceutical primary standard
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Human coagulation factor VIII concentrate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Elizabeth Duncan et al.
Methods in molecular biology (Clifton, N.J.), 992, 321-333 (2013-04-03)
Hemophilia A is an inherited bleeding disorder caused by a deficiency of factor VIII coagulant activity (FVIII:C). Patients are treated with infusions of either plasma-derived or recombinant factor VIII. However, some patients develop inhibitory antibodies (inhibitors) to infused factor VIII
F8 gene and phenotype: single player in a team?
Anna Pavlova
Blood, 121(19), 3784-3785 (2013-05-11)
Factor VIII products and inhibitors in severe hemophilia A.
Alfonso Iorio et al.
The New England journal of medicine, 368(15), 1456-1456 (2013-04-12)
Gouri Shankar Pandey et al.
BioMed research international, 2013, 793502-793502 (2013-04-05)
Flow cytometry is widely used in cancer research for diagnosis, detection of minimal residual disease, as well as immune monitoring and profiling following immunotherapy. Detection of specific host proteins for diagnosis predominantly uses quantitative PCR and western blotting assays. In
S V Ignat'ev et al.
Klinicheskaia laboratornaia diagnostika, (3)(3), 20-22 (2013-07-03)
The sample consisted of 102 patients with hemophilia infected and non-infected with hepatitis viruses. It is established that in case of inhibitory form of hemophilia concentration of IgG increases at the expense of subclass II and in case of non-inhibitory
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service