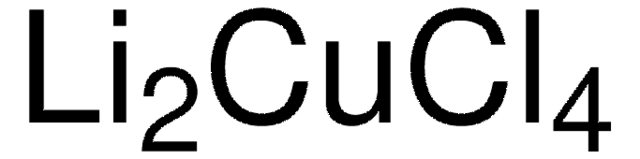82815
Pyridinium p-toluenesulfonate
puriss., ≥99.0% (T)
Synonym(s):
p-Toluenesulfonic acid pyridine salt, PPTS, Pyridine p-toluenesulfonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H13NO3S
CAS Number:
Molecular Weight:
251.30
Beilstein:
3764305
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
puriss.
Quality Level
Assay
≥99.0% (T)
form
crystals
mp
117-120 °C
SMILES string
c1ccncc1.Cc2ccc(cc2)S(O)(=O)=O
InChI
1S/C7H8O3S.C5H5N/c1-6-2-4-7(5-3-6)11(8,9)10;1-2-4-6-5-3-1/h2-5H,1H3,(H,8,9,10);1-5H
InChI key
ZDYVRSLAEXCVBX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Pyridinium p-toluenesulfonate is a mild and efficient catalyst for the formation and cleavage of acetals. This method has been adapted in the completely symmetric synthesis of tetrahydrolipstatin. It is also capable of selectively cleaving tert-butyldimethylsilyl (TBDMS) ethers in the presence of tert-butyldiphenlysilyl (TBDPS) ethers.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R.S. Bhide et al.
Tetrahedron Letters, 27, 671-671 (1986)
L.A. Paquette et al.
Journal of the American Chemical Society, 103, 6526-6526 (1981)
Pyridinium p-toluenesulfonate. A mild and efficient catalyst for the tetrahydropyranylation of alcohols
N. Miyashita et al.
The Journal of Organic Chemistry, 42, 3773-3773 (1977)
L.A. Paquette et al.
The Journal of Organic Chemistry, 46, 3368-3368 (1981)
H. Monti et al.
Synthetic Communications, 13, 1021-1021 (1983)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service