P25507
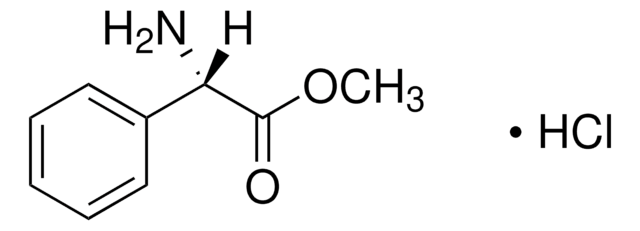
2-Phenylglycine
95%
Synonym(s):
DL-α-Phenylglycine, (±)-α-Aminophenylacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(NH2)COOH
CAS Number:
Molecular Weight:
151.16
Beilstein:
3197862
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to faint yellow
mp
290 °C (subl.) (lit.)
application(s)
detection
SMILES string
NC(C(O)=O)c1ccccc1
InChI
1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)
InChI key
ZGUNAGUHMKGQNY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aaron D Mills et al.
Bioorganic & medicinal chemistry letters, 20(1), 87-91 (2009-12-04)
A developing therapy of cystic fibrosis caused by the DeltaF508 mutation in CFTR employs correction of defective CFTR chloride channel gating by a 'potentiator' and of defective CFTR protein folding by a 'corrector'. Based on SAR data for phenylglycine-type potentiators
Jian-Lian Chen et al.
Analytica chimica acta, 718, 130-137 (2012-02-07)
Three different approaches for immobilizing cross-linked chitosan molecules (CS-s) in sol-gel phases to form chiral OT-CEC capillaries were comparatively investigated in this study. To synthesize column I, a bare capillary was first silanized with triethoxysilane (TEOS) and then reacted with
Motohiro Akazome et al.
The Journal of organic chemistry, 75(3), 660-665 (2010-01-07)
In terms of chiral recognition for racemic aryl methyl sulfoxides in the solid state, three kinds of crystalline (S)-alkylglycyl-(S)-phenylglycines were examined as potential dipeptides host molecules. When (S)-alanyl-(S)-phenylglycines [(S,S)-Ala-Phg] crystallized with aryl methyl sulfoxides, the stereochemistry of preferentially included sulfoxides
Lu Wang et al.
Enzyme and microbial technology, 51(2), 107-112 (2012-06-06)
α-Amino acid ester hydrolases (AEHs) are enzymes of interest to the semi-synthesis of β-lactam antibiotics with α-amino, such as cephalexin and cefaclor. An undesired side reaction, the hydrolysis of α-amino acid ester, had hindered applications in antibiotics synthesis. Although the
Choedchai Saehuan et al.
Biochimica et biophysica acta, 1770(11), 1585-1592 (2007-10-06)
Following induction with D-phenylglycine both d-phenylglycine aminotransferase activity and benzoylformate decarboxylase activity were observed in cultures of Pseudomonas stutzeri ST-201. Induction with benzoylformate, on the other hand, induced only benzoylformate decarboxylase activity. Purification of the benzoylformate decarboxylase, followed by N-terminal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service