All Photos(2)
About This Item
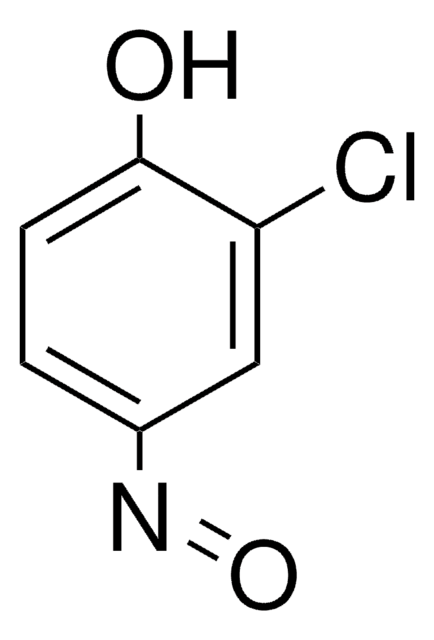
Linear Formula:
CH3C6H4NO
CAS Number:
Molecular Weight:
121.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
72-75 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1ccccc1N=O
InChI
1S/C7H7NO/c1-6-4-2-3-5-7(6)8-9/h2-5H,1H3
InChI key
TWLBRQVYXPMCFK-UHFFFAOYSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S S Hecht et al.
Cancer letters, 20(3), 349-354 (1983-10-01)
3,2'-Dimethyl-4-aminobiphenyl and 3,2'-dimethyl-4-nitrosobiphenyl were administered by subcutaneous injection in peanut oil to 2 groups of 15 male and 15 female Syrian golden hamsters. The total dose of each compound was 5.6 mmol/kg. In the group treated with 3,2'-dimethyl-4-aminobiphenyl, 24 animals
B Kulkarni et al.
Carcinogenesis, 4(10), 1275-1279 (1983-10-01)
High-performance liquid chromatography with electrochemical detection, a highly sensitive and specific method, was used to determine N-hydroxy-o-toluidine and o-toluidine in the urines of male F344 rats after the administration of 0.82 mmol/kg of o-toluidine or o-nitrosotoluene. In a six hour
S S Hecht et al.
Cancer letters, 16(1), 103-108 (1982-05-01)
o-Toluidine hydrochloride and one of its metabolites, o-nitrosotoluene, were administered in the diet (0.028 mol/kg diet) to 2 groups of 30 male F-344 rats for 72 weeks. o-Nitrosotoluene induced significantly more tumors of the bladder (16/30 rats) and liver (20/30)
Y Ohkuma et al.
Archives of biochemistry and biophysics, 372(1), 97-106 (1999-11-24)
Mechanisms of DNA damage by metabolites of carcinogenic o-toluidine in the presence of metals were investigated by the DNA sequencing technique using (32)P-labeled human DNA fragments. 4-Amino-3-methylphenol, a major metabolite, caused DNA damage in the presence of Cu(II). Predominant cleavage
Matthew A Cerny et al.
Archives of biochemistry and biophysics, 436(2), 265-275 (2005-03-31)
The inactivation of cytochrome P450 enzymes by cyclopropylamines has been attributed to a mechanism involving initial one-electron oxidation at nitrogen followed by scission of the cyclopropane ring leading to covalent modification of the enzyme. Herein, we report that in liver
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service