M86804
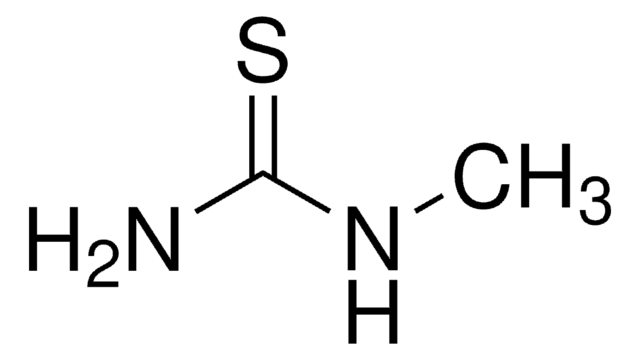
N-Methylurea
97%
Synonym(s):
1-Methylurea, N-Methylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3NHCONH2
CAS Number:
Molecular Weight:
74.08
Beilstein:
878189
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
crystals
SMILES string
CNC(N)=O
InChI
1S/C2H6N2O/c1-4-2(3)5/h1H3,(H3,3,4,5)
InChI key
XGEGHDBEHXKFPX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
L W Yousef et al.
Biochimica et biophysica acta, 984(3), 281-288 (1989-09-18)
Permeability coefficients (P) measured at various penetrant concentrations (C) by the perturbation method can be plotted to distinguish simple diffusion, simple pore kinetics and simple carrier kinetics as follows: for simple diffusion, 1/P = constant; for a simple pore, 1/P
P Vaughan et al.
Carcinogenesis, 12(2), 263-268 (1991-02-01)
Many microorganisms exhibit an adaptive response to mutagenic alkylation damage. In Escherichia coli the response is regulated by the inducible Ada protein. A sensitive immunoassay employing two anti-Ada monoclonal antibodies has been developed here to monitor low levels of induction
Issa Yavari et al.
Molecular diversity, 10(1), 23-27 (2006-01-13)
The stabilized phosphoranes, obtained from the three-component reaction between dialkyl acetylenedicarboxylates and urea or N-methylurea in the presence of triphenylphosphine, undergo a smooth reaction in boiling toluene to produce iminophosphoranes in good yields. Under the same reaction conditions, the phosphorane
M Nagabhushan et al.
Mutation research, 202(1), 163-169 (1988-11-01)
The effects of turmeric extract and its pure yellow pigments curcumin I, II and III were tested on the nitrosation of methylurea by sodium nitrite at pH 3.6 and 30 degrees C. The nitrosomethylurea formed was monitored by checking the
P Ramirez-Victoria et al.
Mutation research, 496(1-2), 39-45 (2001-09-12)
It is known that the poblano green pepper, a significant component in the Mexican diet, contains certain natural compounds such as chlorophyll, beta-carotene, and vitamins, which have antimutagenic and/or anticarcinogenic properties. Using the somatic mutation and recombination test in wing
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service