All Photos(1)
About This Item
Empirical Formula (Hill Notation):
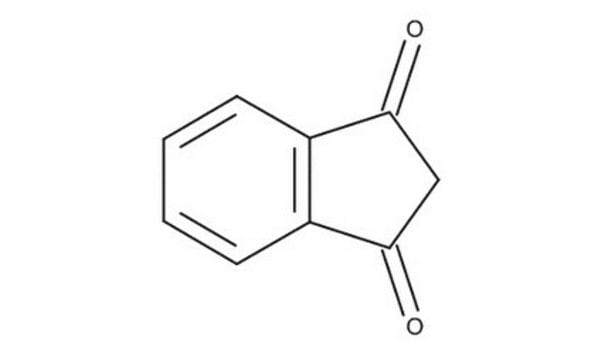
C9H6O2
CAS Number:
Molecular Weight:
146.14
Beilstein:
1210061
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
129-132 °C (lit.)
SMILES string
O=C1CC(=O)c2ccccc12
InChI
1S/C9H6O2/c10-8-5-9(11)7-4-2-1-3-6(7)8/h1-4H,5H2
InChI key
UHKAJLSKXBADFT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Violet Dhayabaran et al.
Luminescence : the journal of biological and chemical luminescence, 32(7), 1339-1348 (2017-05-19)
A novel Schiff base, (S,Z)-4-(methylthio)-2-((3-oxo-2,3-dihydro-1H-inden-1-ylidene)amino)butanoic acid (L) and four M(II) complexes (where M = Co, Cu, Ni and Zn) were synthesized and characterized. The DNA-binding characteristics of the complexes were investigated using various spectroscopic methods and viscosity measurements. Analysis of the results
A Schulman et al.
Journal of the American Veterinary Medical Association, 188(4), 402-405 (1986-02-15)
Five cases of coagulopathy caused by consumption of indanedione (diphacinone)-based rodenticides are reported. In each case, acute onset of lethargy and respiratory distress were the predominant initial clinical signs. Thoracic radiography revealed pulmonary edema, pleural effusion, and/or pericardial effusion as
Abdolmajid Bayandori Moghaddam et al.
Chemical & pharmaceutical bulletin, 54(10), 1391-1396 (2006-10-04)
This is an environmentally friendly method in the field of electroorganic reactions under controlled potential electrolysis, without toxic reagents at a carbon electrode in an undivided cell which involves the (EC) mechanism reaction and comprises two steps alternatively; (i) electrochemical
Bilquees Bano et al.
Bioorganic chemistry, 81, 658-671 (2018-09-27)
Current study deals with the evaluation of indane-1,3-dione based compounds as new class of urease inhibitors. For that purpose, benzylidine indane-1,3-diones (1-30) were synthesized and fully characterized by different spectroscopic techniques including EI-MS, HREI-MS, 1H, and 13C NMR. All synthetic
[Effect of S-oxidation on the anticoagulant effects of 4-hydroxycoumarins, 4-hydroxy-2-pyrones and 1,3-indanediones].
K Rehse et al.
Archiv der Pharmazie, 317(3), 262-267 (1984-03-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service