779385
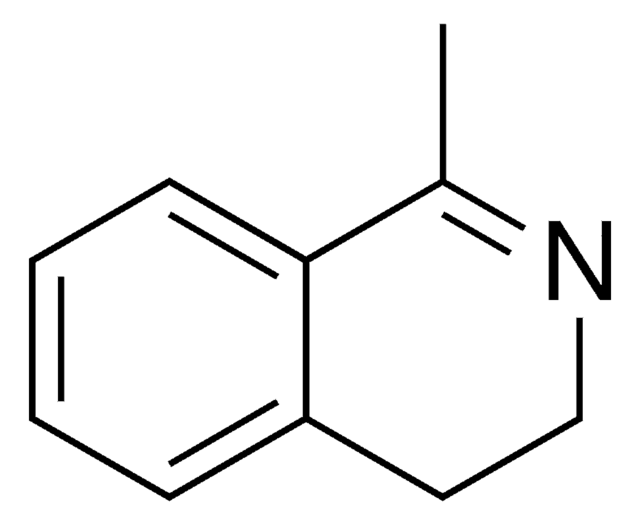
3,4-Dihydroisoquinoline
≥97.5% (GC)
Synonym(s):
3,4-Dihydroisoquinoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9N
CAS Number:
Molecular Weight:
131.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.5% (GC)
97.5-102.5% (T)
form
solid
suitability
complies for identity (IR)
SMILES string
C1Cc2ccccc2C=N1
InChI
1S/C9H9N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-4,7H,5-6H2
InChI key
NKSZCPBUWGZONP-UHFFFAOYSA-N
Application
3,4-Dihydroisoquinoline can be used as a reactant to synthesize:
- 5,6-Dihydro-8H-isoquino[1,2-b]quinazolin-8-one by decarboxylative cyclization reaction with isatoic anhydride using tetrabutylammonium iodide (TBAI).
- 1-naphtholyl tetrahydroisoquinoline by aza-Friedel-Crafts reaction with various naphthols.
- 3,4-dihydroisoquinoline pseudo bases, which are employed as starting materials for the preperation of 3-benzazepine derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solvent-free direct aza-Friedel-Crafts reactions between 3, 4-dihydroisoquinoline and 1-or 2-naphthols
MacLeod PD, et al.
Tetrahedron Letters, 47(38), 6791-6794 (2006)
Electrosynthesis of polycyclic quinazolinones and rutaecarpine from isatoic anhydrides and cyclic amines
Chen Xingyu, et al.
Royal Society of Chemistry Advances, 10(72), 44382-44386 (2020)
A facile, one pot method for the synthesis of 4-acyl-1, 2-dihydro-3-benzazepines, based on the ring expansion of natural and synthetic 3, 4-dihydroisoquinoline pseudo bases
Kartsev VG, et al.
Tetrahedron Letters, 56(50), 6988-6993 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service