All Photos(1)
About This Item
Empirical Formula (Hill Notation):
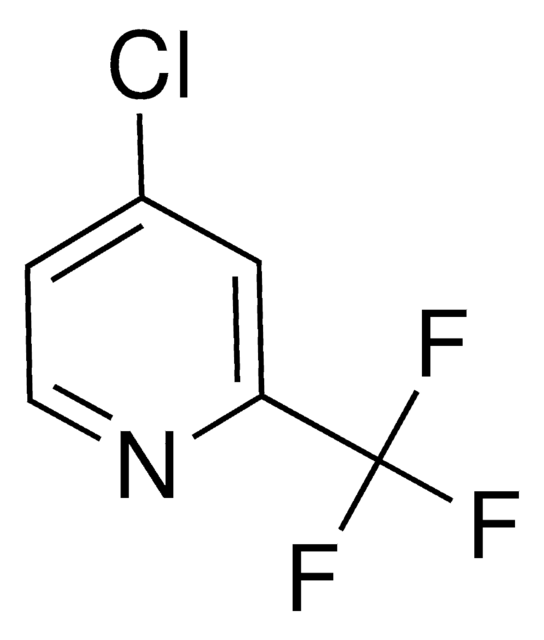
C6H3ClF3N
CAS Number:
Molecular Weight:
181.54
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.4490 (lit.)
bp
146-147 °C (lit.)
density
1.411 g/mL at 25 °C (lit.)
functional group
chloro
fluoro
SMILES string
FC(F)(F)c1ccnc(Cl)c1
InChI
1S/C6H3ClF3N/c7-5-3-4(1-2-11-5)6(8,9)10/h1-3H
InChI key
GBNPVXZNWBWNEN-UHFFFAOYSA-N
General description
2-Chloro-4-(trifluoromethyl)pyridine can be synthesized from 2-chloro-4-iodopyridine.
Application
2-Chloro-4-(trifluoromethyl)pyridine may be used in the synthesis of:
- 4,4′-bis( trifluoromethyl)-2,2′-bipyridine
- 4-(trifluoromethyl)pyridine
- 1,3-bis(4-(trifluoromethyl)pyridin-2-yl)benzene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Cyclometallated platinum (II) complexes of 1, 3-di (2-pyridyl) benzenes: tuning excimer emission from red to near-infrared for NIR-OLEDs.
Rossi E, et al.
Journal of Materials Chemistry, 21(39), 15501-15510 (2011)
Trifluoromethyl-substituted 2, 2'-bipyridine ligands. Synthetic control of excited-state properties of ruthenium (II) tris-chelate complexes.
Furue M, et al.
Inorganic Chemistry, 31(18), 3792-3795 (1992)
Trifluoromethyl-Substituted Pyridines Through Displacement of Iodine by in situ Generated (Trifluoromethyl) copper.
Cottet F and Schlosser M.
European Journal of Organic Chemistry, 2, 327-330 (2002)
The Direct Metalation and Subsequent Functionalization of Trifluoromethyl-Substituted Pyridines and Quinolines.
Schlosser M and Marull M.
European Journal of Organic Chemistry, 8, 1569-1575 (2003)
Wade C Henke et al.
ChemSusChem, 10(22), 4589-4598 (2017-10-13)
We demonstrate that [Cp*Rh] complexes bearing substituted 2,2'-bipyridyl ligands are effective hydrogen evolution catalysts (Cp*=η
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service