All Photos(1)
About This Item
Empirical Formula (Hill Notation):
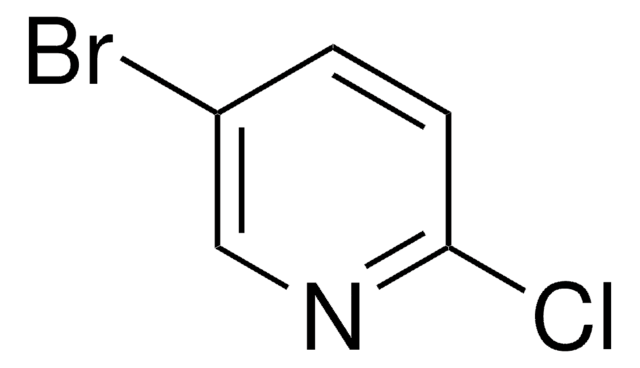
C5H3BrClN
CAS Number:
Molecular Weight:
192.44
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
54-57 °C (lit.)
functional group
bromo
chloro
SMILES string
Clc1ncccc1Br
InChI
1S/C5H3BrClN/c6-4-2-1-3-8-5(4)7/h1-3H
InChI key
HDYNIWBNWMFBDO-UHFFFAOYSA-N
General description
3-Bromo-2-chloropyridine can be synthesized from 3-amino-2-chloropyridine or 2-chloro-3-pyridinamine.
Application
3-Bromo-2-chloropyridine may be used to synthesize:
- acetylenic dipyridone
- 3-ethynyl-2-(phenylmethoxy)-pyridine
- nemertelline
- ortho-chlorodiheteroarylamine4 or 2-chloro-N-(2,3,7-trimethylbenzo[b]thien-6-yl)pyridin-3-amine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Use of hydrogen bonds to control molecular aggregation. behavior of dipyridones and pyridone-pyrimidones designed to form cyclic triplexes.
Boucher E, et al.
The Journal of Organic Chemistry, 60(5), 1408-1412 (1995)
Maria-João R P Queiroz et al.
Bioorganic & medicinal chemistry, 14(20), 6827-6831 (2006-07-18)
ortho-Chlorodiarylamines in the 2,3,7-trimethylbenzo[b]thiophene series were prepared in high yields (70-85%) by C-N palladium-catalyzed cross-coupling using P(t-Bu)(3) as ligand and NaOt-Bu as base. A palladium-assisted C-C intramolecular cyclization of the coupling products gave thienocarbazoles and the dechlorinated diarylamines. Studies of
Synthesis of the first thieno-d-carboline: Fluorescence studies in solution and in lipid vesicles.
Queiroz MJRP, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 181(2), 290-296 (2006)
Alexandre Bouillon et al.
The Journal of organic chemistry, 68(26), 10178-10180 (2003-12-20)
Regioselective and univocal Suzuki cross-coupling reactions performed on halopyridinyl boronic acids provide a flexible and versatile route to a multigram scale synthesis of 2,2'-dichloro-3,4'-bipyridine 14, which allows couplings with excess pyridin-3-yl boronic acid to give a new and efficient two-step
Synthesis of novel halopyridinylboronic acids and esters. Part 2: 2, 4, or 5-Halopyridin-3-yl-boronic acids and esters.
Bouillon A, et al.
Tetrahedron, 58(17), 3323-3328 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)