238198
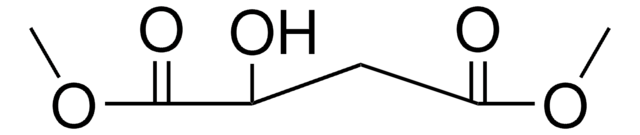
Dimethyl maleate
96%
Synonym(s):
(2Z)-2-Butenedioic acid dimethyl ester, (Z)-2-Butenedioic acid dimethyl ester, (Z)-Dimethyl 2-butenedioate, Dimethyl (Z)-but-2-enedioate
About This Item
Recommended Products
Quality Level
Assay
96%
form
liquid
impurities
≤4% dimethyl fumarate
refractive index
n20/D 1.441 (lit.)
bp
204-205 °C (lit.)
solubility
water: soluble 77.9 g/L at 20 °C
density
1.152 g/mL at 25 °C (lit.)
SMILES string
[H]\C(=C(/[H])C(=O)OC)C(=O)OC
InChI
1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3-
InChI key
LDCRTTXIJACKKU-ARJAWSKDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dimethyl maleate (DMM) is a reactive dienophile and undergoes ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. Mesoporous siliceous SBA-15-supported Cu catalyzed gas phase hydrogenolysis of DMM to 1,4-butanediol (BDO) has been reported. Aluminium chloride has been reported to accelerate the Diels-Alder reaction of DMM and anthracene. DMM can be synthesized by the esterification of maleic anhydride with sulfuric acid and methanol.
Application
- Dissociation of bovine 6S procarboxypeptidase A by reversible condensation with 2,3-dimethyl maleic anhydride: application to the partial characterization of subunit III.: This study explores the dissociation of bovine procarboxypeptidase A using 2,3-dimethyl maleic anhydride, highlighting its applications in the partial characterization of enzyme subunits. The research demonstrates the potential of dimethyl maleate derivatives in protein chemistry and enzyme structure studies. (Puigserver and Desnuelle, 1975).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1 - STOT RE 2 Dermal - STOT SE 3
Target Organs
Respiratory system, Skin
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service