108073
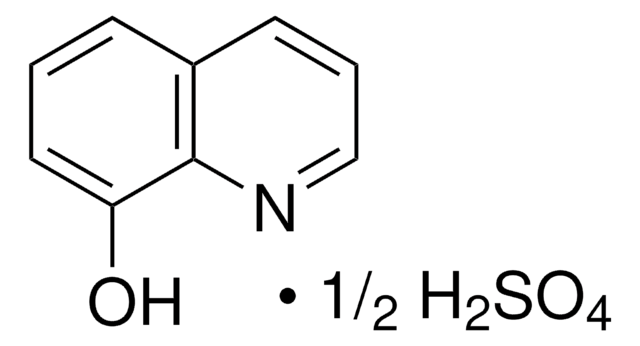
8-Hydroxyquinoline hemisulfate salt hemihydrate
98%
Synonym(s):
8-Quinolinol hemisulfate, 8-Quinolinol sulfate (2:1) monohydrate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C9H7NO · 0.5H2O4S · 0.5H2O
CAS Number:
Molecular Weight:
203.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
176-179 °C (lit.)
solubility
H2O: soluble 100 mg/mL, clear to slightly hazy, yellow to orange
SMILES string
[H]O[H].OS(O)(=O)=O.Oc1cccc2cccnc12.Oc3cccc4cccnc34
InChI
1S/2C9H7NO.H2O4S.H2O/c2*11-8-5-1-3-7-4-2-6-10-9(7)8;1-5(2,3)4;/h2*1-6,11H;(H2,1,2,3,4);1H2
InChI key
BNCXJZDIJIVJJO-UHFFFAOYSA-N
Biochem/physiol Actions
It is a potential copper ligand and is a tested effective proteasome inhibitor.
Preparation Note
8-Hydroxyquinoline hemisulfate salt hemihydrate dissolves in water at a concentration of 100 mg/ml to form a clear to slightly hazy, yellow to yellow-orange coloured solution.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kenyon G Daniel et al.
Biochemical pharmacology, 67(6), 1139-1151 (2004-03-10)
Here we report that organic copper complexes can potently and selectively inhibit the chymotrypsin-like activity of the proteasome in vitro and in vivo. Several copper compounds, such as NCI-109268 and bis-8-hydroxyquinoline copper(II) [Cu(8-OHQ)(2)], can inhibit the chymotrypsin-like activity of purified
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service