N7632
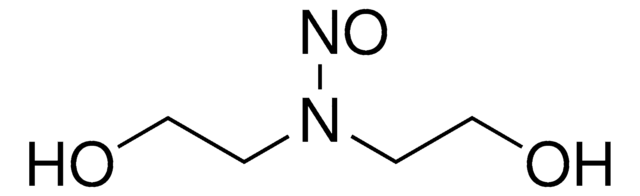
N-Nitrosodiethanolamine
Synonym(s):
2,2′-(Nitrosoimino)bisethanol, N,N-Diethanolnitrosamine, Bis(2-hydroxyethyl)nitrosamine, NDELA
About This Item
Recommended Products
form
liquid
impurities
≤10% Isopropanol
storage temp.
2-8°C
SMILES string
OCCN(N=O)CCO
InChI
1S/C4H10N2O3/c7-3-1-6(5-9)2-4-8/h7-8H,1-4H2
InChI key
YFCDLVPYFMHRQZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Changes in levels of N-nitrosamine formed from amine-containing compounds during chloramination via photocatalytic pretreatment with immobilized TiO(2): Effect of source water and pH.: In this study, NDELA is used as a standard to examine how the formation of nitrosamines, such as N-Nitrosodiethanolamine, is affected by different water sources and pH levels during water treatment processes (Seid et al., 2022).
- Monitoring and risk assessment of hazardous chemicals in toy-slime and putty in the Netherlands.: This research focuses on the presence of N-Nitrosodiethanolamine in children′s toys, providing important data for consumer safety and regulatory standards (Braver et al., 2021).
- On-line solid phase extraction-ultra-high performance liquid chromatography coupled to tandem mass spectrometry for the determination of N-nitrosodiethanolamine in baby shampoo.: Highlights the usage of N-Nitrosodiethanolamine as an analytical standard in application of advanced chromatographic techniques for detecting harmful substances in consumer products, ensuring product safety and regulatory compliance (Tada et al., 2021).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
52.9 °F
Flash Point(C)
11.6 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service