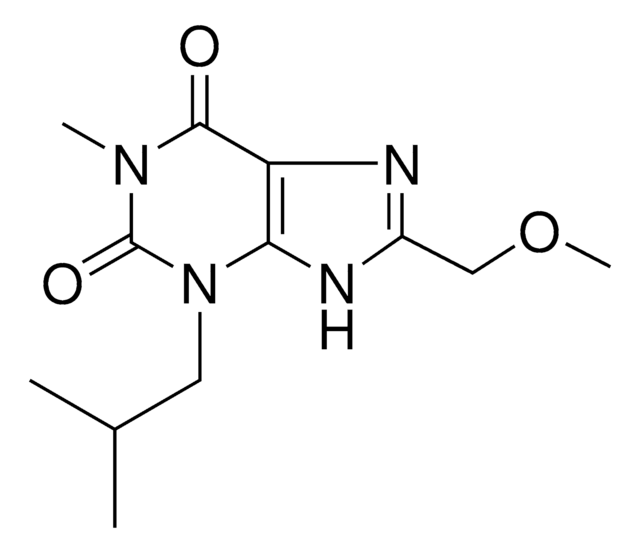
The solubility instructions can be found in the Product Information Sheet as follows:
IBMX is soluble in different organic solvents, as follows:
Warm methanol at 50 mg/mL
Ethanol at 10 mg/mL. Dissolves at 25 mg/mL only with sonication.(Schwertner, H.A. et al., Analyt. Chem., 48(13), 1875-1878 (1976))
Various publications report preparation in DMSO at 50 mg/mL (Liu, L., and Keefe, D.L., Methods Mol. Biol., 371, 191-207 (2007).) and at 110 mg/mL (Eckel, J., The Cellular Secretome and Organelle Crosstalk. Academic Press/Elsevier, p. 167 (2018)).
A 10 mM aqueous solution can be prepared by warming in a boiling water bath.
Please access the Product Information Sheet below:
https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/116/537/i7018pis.pdf