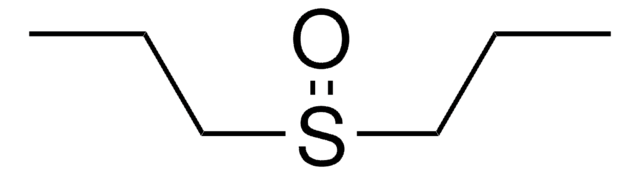P35405
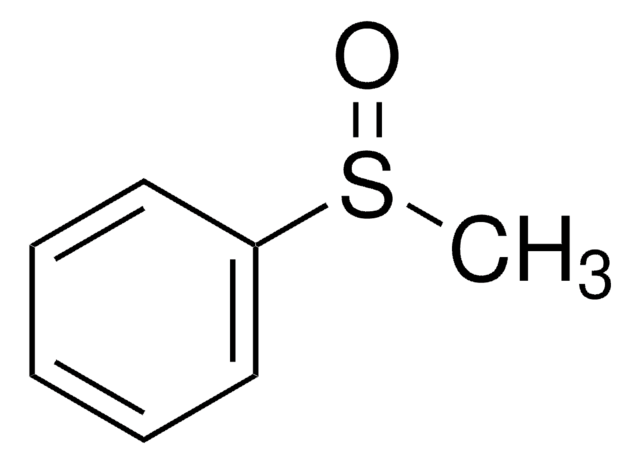
Diphenyl sulfoxide
96%
Synonym(s):
Phenyl sulfoxide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(C6H5)2SO
CAS Number:
Molecular Weight:
202.27
Beilstein:
1908444
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
crystals
bp
206-208 °C/13 mmHg (lit.)
mp
69-71 °C (lit.)
SMILES string
O=S(c1ccccc1)c2ccccc2
InChI
1S/C12H10OS/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
InChI key
JJHHIJFTHRNPIK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Preparation of radiochemicals: Diphenyl sulfoxide plays a role in the synthesis of [(11)C]cyanide from [(11)C]methyl iodide, facilitating rapid and efficient production of radiochemicals for medical imaging applications (Kikuchi et al., 2022).
- Catalytic oxidation processes: The photocatalytic and catalytic oxidation of diphenyl sulphide to sulfoxide and sulfone was examined, highlighting the effectiveness of hydrogen peroxide and TiO2 polymorphs in optimizing chemical processes (Mikrut et al., 2022).
- Dielectric properties research: The study on dielectric properties of high organic sulfur coal highlighted the modeling of sulfur compounds, which could include diphenyl sulfoxide, enhancing our understanding of materials science in energy sectors (Cai et al., 2019).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
David Crich et al.
Organic letters, 8(5), 959-962 (2006-02-24)
The formation of sialic acid glycosides with a thiosialic acid derivative, diphenyl sulfoxide, and trifluoromethanesulfonic anhydride is reported. With an excess of diphenyl sulfoxide, glycal formation can be completely suppressed and excellent yields are obtained for coupling to a wide
Deju Ye et al.
The Journal of organic chemistry, 74(4), 1733-1735 (2009-01-20)
An efficient approach to the dehydrative sialylation of various substrates with C-4-aminated sialyl-hemiketal donors by using the reagent combination of diphenyl sulfoxide and triflic anhydride is reported. By using a C-4-hindered non-nucleophilic amine auxiliary, excellent yields and high alpha-stereoselectivities were
S C Lee et al.
Biochemical pharmacology, 49(11), 1567-1576 (1995-05-26)
The caecal microflora from female rats show a greater ability to reduce the sulphoxide group of sulindac than either the liver or kidneys. Studies on sulphoxide reduction by Escherichia coli showed that NADH, NADPH and dithiothreitol (DTT), but not acetaldehyde
Ya-Juan Wang et al.
Carbohydrate research, 346(11), 1271-1276 (2011-05-31)
An N-acetyl-5-N,4-O-oxazolidinone-protected p-toluene 2-thio-sialoside donor, promoted by Ph(2)SO/Tf(2)O/TTBPy, was thoroughly investigated in the coupling to various acceptors. The stereoselectivity of the sialylation was found to be dependent on the various reaction conditions, such as pre-activation time, reaction time, the amount
Lu Liu et al.
Chemical reviews, 119(24), 12422-12490 (2019-12-14)
More than 50 years have passed since Haszeldine reported the first addition of a trifluoromethyl radical to an allene; in the intervening years, both the chemistry of allenes and the reactivity of single-electron species have become topics of intense interest.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service