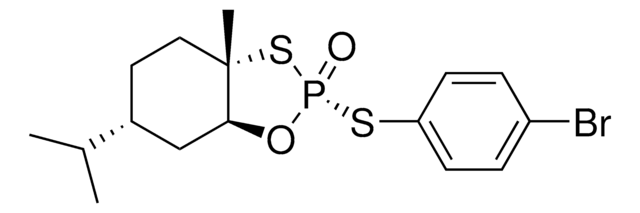
ALD00616
Ethyl (R,E)-2-((mesitylsulfinyl)imino)acetate
≥95%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C13H17NO3S
CAS Number:
Molecular Weight:
267.34
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder
reaction suitability
reaction type: C-C Bond Formation
mp
62-65 °C
storage temp.
−20°C
Related Categories
Application
Ethyl (R,E)-2-((mesitylsulfinyl)imino)acetate is a chiral glyoxylate-derived sulfinimine reagent used for the synthesis of optically pure amino acids through a decarboxylative radical cross-coupling reaction.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shengyang Ni et al.
Angewandte Chemie (International ed. in English), 57(44), 14560-14565 (2018-09-14)
The direct union of primary, secondary, and tertiary carboxylic acids with a chiral glyoxylate-derived sulfinimine provides rapid access into a variety of enantiomerically pure α-amino acids (>85 examples). Characterized by operational simplicity, this radical-based reaction enables the modular assembly of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service