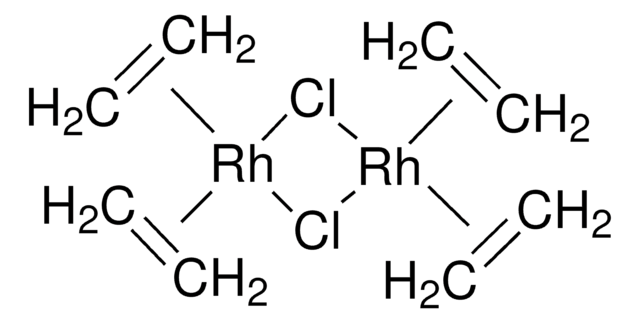
683132
Chiralyst P493
Umicore, 98%
Synonym(s):
Chloro(1,5-cyclooctadiene)rhodium(I) dimer, Di(μ-chloro)bis(1,5-cyclooctadiene)dirhodium(I), 1,5-Cyclooctadienerhodium(I) chloride dimer, Bis(1,5-cyclooctadiene)dirhodium(I) dichloride, Di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]dirhodium, Rhodium(I) chloride 1,5-Cyclooctadiene complex dimer, [Rh(COD)Cl]2
About This Item
Recommended Products
Assay
98%
form
crystals
reaction suitability
core: rhodium
reaction type: Cross Couplings
reagent type: catalyst
mp
216.3-219.2 °C
243 °C (dec.) (lit.)
SMILES string
Cl[Rh].Cl[Rh].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.2ClH.2Rh/c2*1-2-4-6-8-7-5-3-1;;;;/h2*1-2,7-8H,3-6H2;2*1H;;/q;;;;2*+1/p-2/b2*2-1-,8-7-;;;;
InChI key
QSUDXYGZLAJAQU-MIXQCLKLSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Efficient and Selective Catalysts for Asymmetric Synthesis
- Employed for the synthesis of rhodium complex of heterocyclic carbenes (NHCs).
- Modified and coated on the surface of ferrite magnetic nanoparticles for catalyzing hydroformylation reaction of olefins.
- Used for the synthesis of triple-layer structure to be used as a ring-opening polymerization catalyst.
- As an effective catalyst for dehydrogenation of amine-borane adducts (hydrogen storage materials) such as ammonia-borane.
Legal Information
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Precatalysts for asymmetric catalysis offer batch-to-batch consistency in a range of catalytic reactions, supporting diverse research needs.
Precatalysts for asymmetric catalysis offer batch-to-batch consistency in a range of catalytic reactions, supporting diverse research needs.
Precatalysts for asymmetric catalysis offer batch-to-batch consistency in a range of catalytic reactions, supporting diverse research needs.
Precatalysts for asymmetric catalysis offer batch-to-batch consistency in a range of catalytic reactions, supporting diverse research needs.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer 96%](/deepweb/assets/sigmaaldrich/product/structures/700/585/b2e5ae1d-2b88-42c8-a071-ef828d4a104c/640/b2e5ae1d-2b88-42c8-a071-ef828d4a104c.png)