All Photos(1)
About This Item
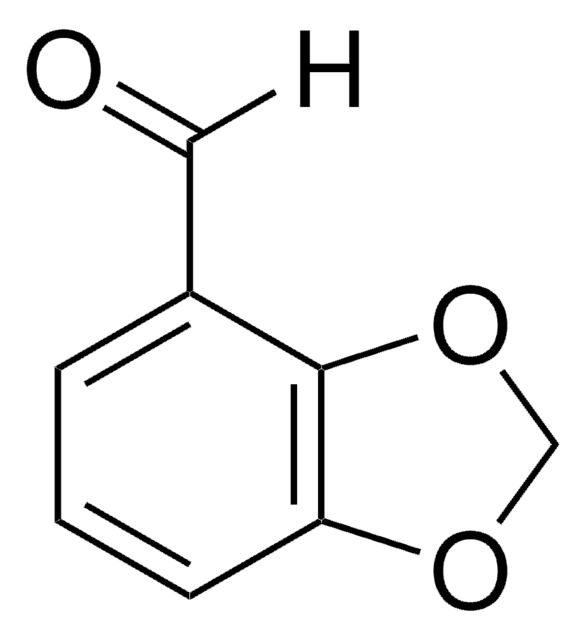
Empirical Formula (Hill Notation):
C9H8O3
CAS Number:
Molecular Weight:
164.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
105 °C/15 mmHg (lit.)
mp
50-52 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccc2OCCOc2c1
InChI
1S/C9H8O3/c10-6-7-1-2-8-9(5-7)12-4-3-11-8/h1-2,5-6H,3-4H2
InChI key
CWKXDPPQCVWXAG-UHFFFAOYSA-N
Application
1,4-Benzodioxan-6-carboxaldehyde has been used:
- as building block in the synthesis of tetrahydroisoquinolinones
- in the preparation of benzofuran analog, a potential inhibitor of CAMP-specific phosphodiesterase type IV
- in the synthesis of (5Z)-5-(2,3-dihydro-1,4-benzodioxan-6-ylmethylene)-1-methyl-2-thioxoimidazolidin-4-one
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Khadidja Bourahla et al.
Molecules (Basel, Switzerland), 16(9), 7377-7390 (2011-09-01)
A practical protocol for the preparation of (5Z)-2-alkylthio-5-arylmethylene-1-methyl-1,5-dihydro-4H-imidazol-4-one derivatives is reported. The new compounds were obtained in good yield and stereoselectivity in two steps, namely a solvent-free Knoevenagel condensation under microwave irradiation, followed by an S-alkylation reaction with various halogenoalkanes.
Structure-activity relationships involving the catechol subunit of rolipram.
Stafford JA, et al.
Bioorganic & Medicinal Chemistry Letters, 4(15), 1855-1860 (1994)
M Lebl
Bioorganic & medicinal chemistry letters, 9(9), 1305-1310 (1999-05-26)
A new technique for high throughput solid phase synthesis using the centrifuge based liquid removal from readily available standard microtiterplates is described. This technique eliminates the filtration step and is therefore applicable to simultaneous processing of an unlimited number of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)