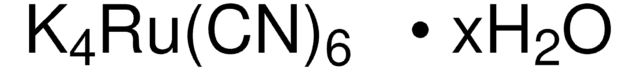208019
Potassium hexacyanoferrate(III)
99%
Synonym(s):
Potassium ferricyanide, Red prussiate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
K3Fe(CN)6
CAS Number:
Molecular Weight:
329.24
EC Number:
MDL number:
UNSPSC Code:
12352103
Recommended Products
Assay
99%
SMILES string
[K+].[K+].[K+].N#C[Fe-3](C#N)(C#N)(C#N)(C#N)C#N
InChI
1S/6CN.Fe.3K/c6*1-2;;;;/q;;;;;;-3;3*+1
InChI key
MIMJFNVDBPUTPB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2
Supplementary Hazards
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Anna S Tarasova et al.
Environmental toxicology and chemistry, 30(5), 1013-1017 (2011-02-11)
The current study deals with the effect of humic substances (HS) on toxicity of solutions of a model inorganic oxidizer, potassium ferricyanide. Chemical reactions responsible for toxicity changes are under consideration. The bioluminescent system of coupled enzymatic reactions catalyzed by
Daehee Kim et al.
ChemSusChem, 5(6), 1086-1091 (2012-05-10)
To scale-up microbial fuel cells (MFCs), installing multiple unit cells in a common reactor has been proposed; however, there has been a serious potential drop when connecting unit cells in series. To determine the source of the loss, a basic
Lu Han et al.
Journal of pharmaceutical and biomedical analysis, 58, 141-145 (2011-10-15)
In this paper, a novel chemiluminescence (CL) system, 2-phenyl-4, 5-di (2-furyl) imidazole (PDFI)-potassium ferricyanide, for the determination of terbutaline sulfate was described. The method was based on enhancement of CL emission of PDFI-potassium ferricyanide system in the presence of terbutaline
Hideo Takakusa et al.
Drug metabolism and disposition: the biological fate of chemicals, 39(6), 1022-1030 (2011-03-03)
Lapatinib, an oral breast cancer drug, has recently been reported to be a mechanism-based inactivator of cytochrome P450 (P450) 3A4 and also an idiosyncratic hepatotoxicant. It was suggested that formation of a reactive quinoneimine metabolite was involved in mechanism-based inactivation
Liju Yang et al.
Biomedical microdevices, 13(2), 279-289 (2010-11-26)
Microlithographically fabricated interdigitated microsensor electrodes (IMEs) were cleaned, surface activated, chemically functionalized (amine) and derivatized with an Acrloyl-PEG-NHS to receive a spun-applied monomer cocktail of UV polymerizable monomer. IMEs were 2050.5, 1550.5, 1050.5 and 0550.5 possessing lines and spaces that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service