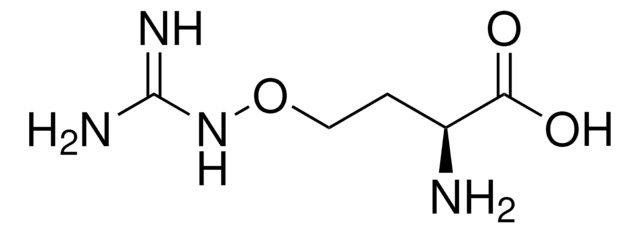
A9834
2-Amino-5,6-dihydro-6-methyl-4H-1,3-thiazine
≥98%
Synonym(s):
AMT
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H10N2S · HCl
CAS Number:
Molecular Weight:
166.67
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic (organic)
Assay
≥98%
form
powder
solubility
water: 25 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
Cl[H].CC1CCN=C(N)S1
InChI
1S/C5H10N2S.ClH/c1-4-2-3-7-5(6)8-4;/h4H,2-3H2,1H3,(H2,6,7);1H
InChI key
HVJCRMIQAMEJNM-UHFFFAOYSA-N
Gene Information
human ... FPGS(2356)
Biochem/physiol Actions
As a selective type II (inducible) nitric oxide synthase (NOS) inhibitor, AMT was tested against LPS-induced inflammation in rats.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W R Tracey et al.
Canadian journal of physiology and pharmacology, 73(5), 665-669 (1995-05-01)
Selective type II (inducible) nitric oxide synthase (NOS) inhibitors have several potential therapeutic applications, including treatment of sepsis, diabetes, and autoimmune diseases. The ability of two novel, selective inhibitors of type II NOS, S-ethylisothiourea (EIT) and 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine (AMT), to inhibit
M Nakane et al.
Molecular pharmacology, 47(4), 831-834 (1995-04-01)
We have identified two novel potent and selective inhibitors of inducible nitric oxide synthase, S-ethylisothiourea and 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine. Ki values of 14.7 nM for S-ethylisothiourea and 4.2 nM for 2-amino-5,6-dihydro-6-methyl-4H-1,3-thiazine were obtained with partially purified preparations of inducible nitric oxide synthase.
Axelle Cooreman et al.
International journal of molecular sciences, 23(9) (2022-05-15)
Connexin43 (Cx43) hemichannels form a pathway for cellular communication between the cell and its extracellular environment. Under pathological conditions, Cx43 hemichannels release adenosine triphosphate (ATP), which triggers inflammation. Over the past two years, azithromycin, chloroquine, dexamethasone, favipiravir, hydroxychloroquine, lopinavir, remdesivir
Ju Hee Lee et al.
Archives of pharmacal research, 38(7), 1304-1311 (2015-03-07)
The aerial parts of Houttuynia cordata used for treating inflammation-related disorders contain flavonoids as major constituents. Since certain flavonoids possess anti-inflammatory activity, especially in the lung, the pharmacological activities of H. cordata and the flavonoid constituents were evaluated using in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service