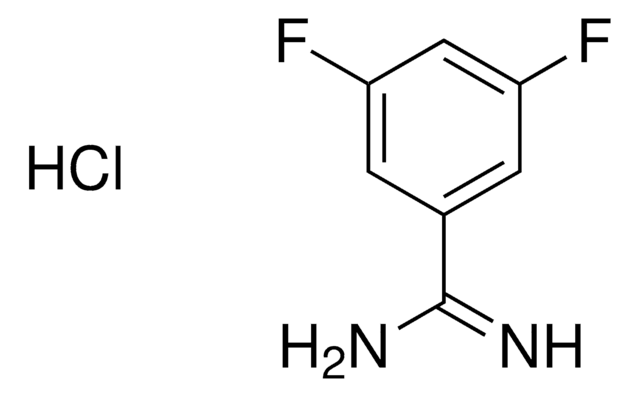
63226
Benzamidine hydrochloride 1 M solution
Synonym(s):
Benzamidine hydrochloride solution, Additive Screening Solution 37/Kit-No 78374
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352202
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
storage temp.
2-8°C
SMILES string
Cl.NC(=N)c1ccccc1
InChI
1S/C7H8N2.ClH/c8-7(9)6-4-2-1-3-5-6;/h1-5H,(H3,8,9);1H
InChI key
LZCZIHQBSCVGRD-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ryan Walsh et al.
Integrative biology : quantitative biosciences from nano to macro, 3(12), 1197-1201 (2011-11-01)
Enzyme inhibitors are usually classified as competitive, non-competitive or mixed non-competitive. Each of these designations has a serious limitation in that it only describes an extreme of inhibitory behaviour. The non-competitive inhibition equation only considers an approach to complete inhibition
Dian Jiao et al.
Journal of computational chemistry, 30(11), 1701-1711 (2009-04-29)
We have calculated the binding free energies of a series of benzamidine-like inhibitors to trypsin with a polarizable force field using both explicit and implicit solvent approaches. Free energy perturbation has been performed for the ligands in bulk water and
Yu Cheng Zhu et al.
Pest management science, 68(5), 692-701 (2012-01-10)
The potential development of resistance to Bacillus thuringiensis (Bt) cotton and surging of non-targeted insects is a major risk in the durability of Bt plant technology. Midgut proteinases are involved in Bt activation and degradation. Proteinase inhibitors may be used
Joseph Vamecq et al.
European journal of medicinal chemistry, 45(7), 3101-3110 (2010-04-30)
Five bis-benzamidines were screened towards murine magnesium deficiency-dependent audiogenic seizures, unravelling two compounds with efficacious doses 50 (ED(50)) less than 10mg/kg. They were also screened against maximal electroshock and subcutaneous pentylenetetrazole-induced seizures, and explored for superoxide -scavenging activity. 1,2-Ethane bis-1-amino-4-benzamidine
Amy Capes et al.
Bioorganic & medicinal chemistry, 20(4), 1607-1615 (2012-01-24)
Quinols have been developed as a class of potential anti-cancer compounds. They are thought to act as double Michael acceptors, forming two covalent bonds to their target protein(s). Quinols have also been shown to have activity against the parasite Trypanosoma
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service