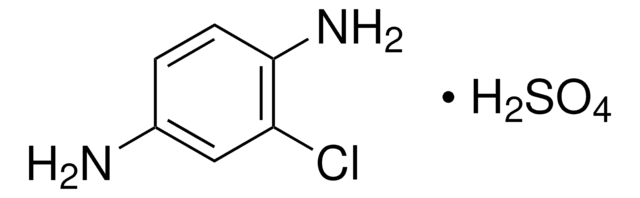
N21200
2-Nitro-1,4-phenylenediamine
95%
Synonym(s):
1,4-Diamino-2-nitrobenzene, 2-Nitro-p-phenylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H3(NH2)2
CAS Number:
Molecular Weight:
153.14
Beilstein:
2210195
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
powder
mp
135-138 °C (lit.)
SMILES string
Nc1ccc(N)c(c1)[N+]([O-])=O
InChI
1S/C6H7N3O2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H,7-8H2
InChI key
HVHNMNGARPCGGD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
>372.2 °F - Pensky-Martens closed cup
Flash Point(C)
> 189 °C - Pensky-Martens closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J J Yourick et al.
Toxicology and applied pharmacology, 166(1), 13-23 (2000-06-30)
2-Nitro-p-phenylenediamine (2NPPD) is a dye used in semipermanent and permanent (tinting color) hair dye formulations. National Toxicology Program toxicology and carcinogenesis testing of 2NPPD has raised concerns about its safety. Therefore, we initiated in vitro studies to measure absorption and
M Nakao et al.
Chemical & pharmaceutical bulletin, 39(1), 177-180 (1991-01-01)
Reductive metabolism of the hair dye constituent, nitro-p-phenylenediamine (2-nitro-1,4-diaminobenzene, NPDA), and its acetylated metabolite, NPDA N4-acetate, was investigated with rat liver subcellular fractions, microsomes and cytosol. Under anaerobic conditions, these compounds were reduced to their corresponding amines by these fractions.
M Nakao et al.
Journal of toxicology and environmental health, 11(1), 93-100 (1983-01-01)
The distribution, excretion, and metabolism of nitro-p-phenylenediamine, a constituent of hair dye, was studied after administration of [14C]nitro-p-phenylene-diamine (2.6 mg/30 microCi/kg) to male rats. After intraperitoneal administration, 37.4% of the radioactivity administered was excreted in the urine and 54.3% in
S Grégoire et al.
Skin pharmacology and physiology, 21(2), 89-97 (2008-01-12)
Percutaneous penetration studies are usually performed in human skin samples set up in a Franz cell device. The ability to perform these studies may depend on the availability of skin samples. Reconstructed skin models are an interesting alternative to overcome
In vivo skin penetration studies of 2,4-toluenediamine, 2,4-diaminoanisole, 2-nitro-p-phenylenediamine, p-dioxane and N-nitrosodiethanolamine in cosmetics.
F N Marzulli et al.
Food and cosmetics toxicology, 19(6), 743-747 (1981-12-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service