All Photos(4)
About This Item
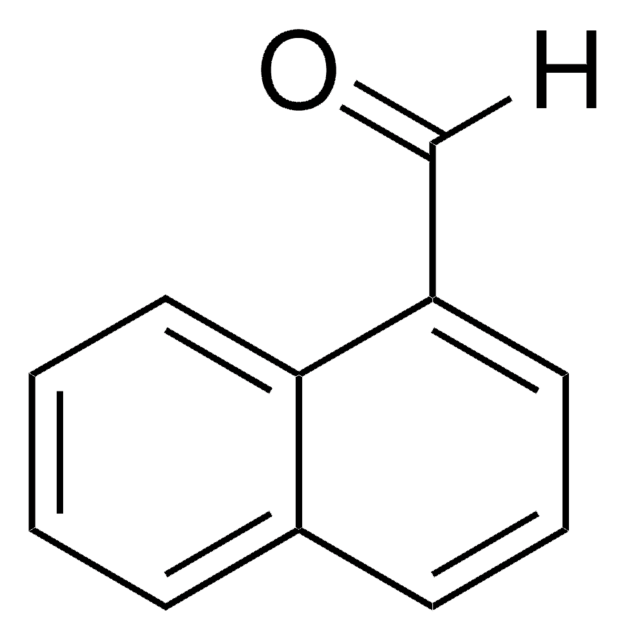
Linear Formula:
C10H7CHO
CAS Number:
Molecular Weight:
156.18
Beilstein:
507750
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
58-61 °C (lit.)
storage temp.
−20°C
SMILES string
[H]C(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O/c12-8-9-5-6-10-3-1-2-4-11(10)7-9/h1-8H
InChI key
PJKVFARRVXDXAD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Naphthaldehyde can be used as a reactant:
- In proline catalyzed aldol reaction.
- In asymmetric three-component Mannich reaction.
- For the synthesis of Hantzsch 1,4-dihydropyridines.13}
- Asymmetric benzoin condensation reaction.
- For the synthesis of pyrazolo[1,2−b]phthalazinediones.
- For the synthesis C60 by flash vacuum pyrolysis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Proline-catalyzed direct asymmetric aldol reactions.
List B, et al.
Journal of the American Chemical Society, 122(10), 2395-2396 (2000)
Zhipeng Zhang et al.
Nature communications, 7, 12478-12478 (2016-08-18)
Due to the high versatility of chiral cyanohydrins, the catalytic asymmetric cyanation reaction of carbonyl compounds has attracted widespread interest. However, efficient protocols that function at a preparative scale with low catalyst loading are still rare. Here, asymmetric counteranion-directed Lewis
The direct catalytic asymmetric three-component Mannich reaction.
List B
Journal of the American Chemical Society, 122(38), 9336-9337 (2000)
Amelioration of H4 [W12SiO40] by nanomagnetic heterogenization: For the synthesis of 1H-pyrazolo [1, 2-b] phthalazinedione derivatives.
Arora P and Rajput JK
Applied Organometallic Chemistry, 32(2), e4001-e4001 (2018)
Dong-Sheng Lee et al.
Chirality, 28(1), 65-71 (2015-10-22)
Chiral O,N,O-tridentate phenol ligands bearing a camphor backbone were found to be effective chiral catalysts for the enantioselective addition of diethylzinc to aromatic aldehydes, resulting in high enantioselectivities (80-95% ee) at room temperature.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service