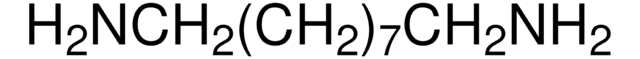D16401
1,12-Diaminododecane
98%
Synonym(s):
1,12-Dodecanediamine, Dodecamethylenediamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)12NH2
CAS Number:
Molecular Weight:
200.36
Beilstein:
1742765
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
flakes
mp
67-69 °C (lit.)
SMILES string
NCCCCCCCCCCCCN
InChI
1S/C12H28N2/c13-11-9-7-5-3-1-2-4-6-8-10-12-14/h1-14H2
InChI key
QFTYSVGGYOXFRQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Feedstock for polymer synthesis. Source of twelve carbon chain for medicinal drugs.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B - Skin Sens. 1
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
311.0 °F - closed cup
Flash Point(C)
155 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Hochreiter et al.
Naunyn-Schmiedeberg's archives of pharmacology, 361(3), 235-246 (2000-03-24)
A series of diamines with the general structure NH2(CH2)xNH2, x=2-12, was tested for their potential effects on cell proliferation of cultured rat C6 glioma cells in comparison to natural polyamines. Long chain diamines reduced cell number after 48 h in
Michelle J Pisani et al.
Dalton transactions (Cambridge, England : 2003), 39(8), 2078-2086 (2010-02-12)
A simpler method for the purification of cucurbit[10]uril (Q[10]) from the Q[10].Q[5] inclusion complex is reported. 1,12-Diaminododecane was used to displace Q[5], as opposed to the synthetic melamine derivative currently used. The binding of trans-[{PtCl(NH(3))(2)}(2)(micro-NH(2)(CH(2))(8)NH(2))](2+) (CT008) and [{Ru(phen)(2)}(2)(micro-bb(5))](4+) {phen =
Andrew R Hirst et al.
Langmuir : the ACS journal of surfaces and colloids, 20(25), 10851-10857 (2004-12-01)
The self-assembly of diaminododecane with dendritic l-lysine-based peptides to form gel-phase materials was investigated in a range of different solvents. The degree of structuring was modulated by the solvent employed, an effect which induced subtle changes in the mesoscale aggregate
Zhong-Xiu Chen et al.
The journal of physical chemistry. B, 115(8), 1798-1806 (2010-12-31)
Molecular recognition by means of multiple hydrogen bonds is of great importance in biological functions. In this paper, an orotic acid derived bolaamphiphile 1,12-diaminododecane diorotate (DDO) with molecular recognition function moieties was designed. Both self-aggregation behavior and molecular recognition with
Kangkyun Baek et al.
Chemical communications (Cambridge, England), 46(23), 4091-4093 (2010-05-19)
The X-ray crystal structure of 1,12-dodecane diammonium (C(12)DA(2+)) encapsulated in cucurbit[8]uril reveals an unconventional U-shaped conformation of C(12)DA(2+), which is attributed to the favorable host-guest interactions to overcome the charge-charge repulsion of the two ammonium groups in close proximity and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service