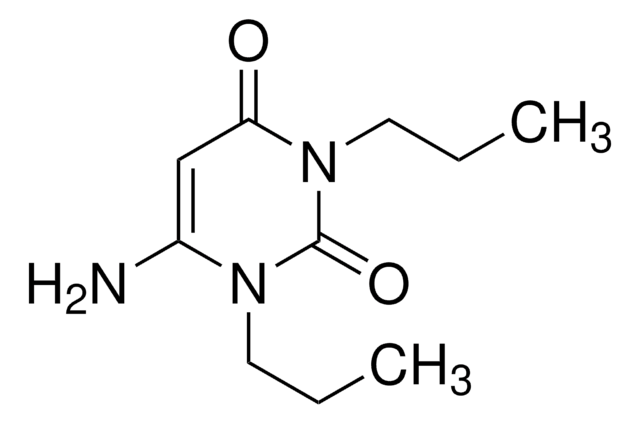
A50606
6-Aminouracil
97%
Synonym(s):
4-Amino-2,6-dihydroxypyrimidine, 6-Amino-2,4-pyrimidinediol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H5N3O2
CAS Number:
Molecular Weight:
127.10
Beilstein:
120491
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
≥360 °C (lit.)
SMILES string
Nc1cc(O)nc(O)n1
InChI
1S/C4H5N3O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H4,5,6,7,8,9)
InChI key
LNDZXOWGUAIUBG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Hirota et al.
Nucleic acids symposium series, (37)(37), 59-60 (1997-01-01)
Inhibitors of thymidine phosphorylase (dThdPase) are expected to suppress the growth and metastasis of tumor cells by inhibition of angiogenesis and were designed by utilizing the three dimensional structure of the enzyme. 5-Substituted 6-aminouracils (5) and 7-substituted pyrrolo[2,3-d]pyrimidine-2,4-diones (6) were
María-Jesús Pérez-Pérez et al.
Mini reviews in medicinal chemistry, 5(12), 1113-1123 (2005-12-27)
Thymidine Phosphorylase (TPase) catalyses the reversible phosphorolysis of pyrimidine 2'-deoxynucleosides to 2-deoxyribose-1-phosphate and their respective pyrimidine bases, including the phosphorolysis of nucleoside analogues with important antiviral or anticancer properties. Moreover, TPase, identified also as the angiogenic platelet-derived endothelial cell growth
Christine Gersch et al.
Nucleosides, nucleotides & nucleic acids, 27(8), 967-978 (2008-08-13)
The 1980 identification of nitric oxide (NO) as an endothelial cell-derived relaxing factor resulted in an unprecedented biomedical research of NO and established NO as one of the most important cardiovascular, nervous and immune system regulatory molecule. A reduction in
Kyung Mee Kim et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 877(1-2), 65-70 (2008-12-17)
Uric acid (UA) can be directly converted to allantoin enzymatically by uricase in most mammals except humans or by reaction with superoxide. UA can react directly with nitric oxide to generate 6-aminouracil and with peroxynitrite to yield triuret; both of
H Sladowska et al.
Acta poloniae pharmaceutica, 53(1), 39-46 (1996-01-01)
Synthesis of 6-substituted 2,4-dioxo-1,2,3,4,5,6,7,8-octahydropyrimido[4,5-d]pyrimidines [III-VI] obtained by cyclocondensation of 1-phenyl-6-aminouracil with formaline and the primary amines is described. Compounds III, V, VI in the Mannich reaction with secondary cyclic amines yield the corresponding 3-substituted N-aminomethyl derivatives VII-X. Some of them
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service