All Photos(1)
About This Item
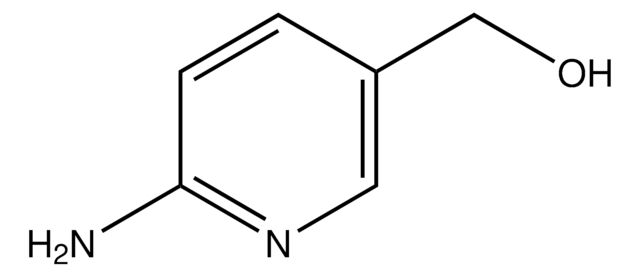
Empirical Formula (Hill Notation):
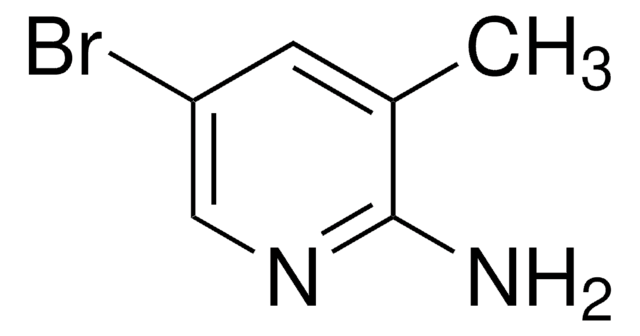
C5H6BrN3
CAS Number:
Molecular Weight:
188.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
155 °C (dec.) (lit.)
functional group
bromo
SMILES string
Nc1cc(Br)cnc1N
InChI
1S/C5H6BrN3/c6-3-1-4(7)5(8)9-2-3/h1-2H,7H2,(H2,8,9)
InChI key
YRGMYJUKFJPNPD-UHFFFAOYSA-N
General description
2,3-Diamino-5-bromopyridine can be prepared from 2-amino-3-nitro-5-bromopyridine via reduction using stannous chloride.
Application
2,3-Diamino-5-bromopyridine may be used in the preparation of the following heterocyclic compounds:
- 6-bromoimidazo-[b]pyridine
- 11-bromopyrido[2′,3′:5,6]pyrazino[2,3-f][1,10]phenanthroline
- 6-bromo-3-(tetrahydro-2H-pyran-2-yl)-3H-imidazo[4,5-b]pyridine
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metabolite analogs. VIII. Syntheses of some imidazopyridines and pyridotriazoles.
Graboyes H and Day AR.
Journal of the American Chemical Society, 79(24), 6421-6426 (1957)
Re (I) Complexes of Substituted dppz: A Computational and Spectroscopic Study.
van der Salm H, et al.
Inorganic Chemistry, 53(6), 3126-3140 (2014)
Microwave-Assisted C-2 Direct Alkenylation of Imidazo [4, 5-b] pyridines: Access to Fluorescent Purine Isosteres with Remarkably Large Stokes Shifts.
Baladi T, et al.
European Journal of Organic Chemistry, 2016(14), 2421-2434 (2016)
Magda A Akl et al.
Dalton transactions (Cambridge, England : 2003), 49(44), 15769-15778 (2020-11-05)
Worldwide, prostate cancer is considered to be one of the three most commonly occurring cancers amongst the male population. Clinically, early detection of diverse forms of cancer before they spread and become incurable plays an important role in treatment strategy.
Sundaram Ellairaja et al.
Biosensors & bioelectronics, 91, 82-88 (2016-12-20)
Bilirubin, a key biomarker for the jaundice and its clinical diagnosis needs a better analytical tool. A novel and simple fluorescent platform based on (2,2'-((1E,1'E)-((6-bromopyridine-2,3-diyl) bis(azanylylidene)) bis(methanylylidene diphenol) (BAMD) was designed. BAMD showed a remarkable fluorescent intensity with a very
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service