All Photos(2)
About This Item
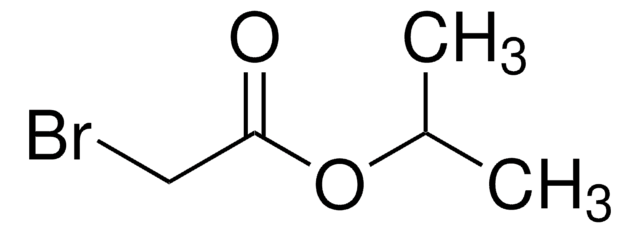
Linear Formula:
BrCH2CO2C6H5
CAS Number:
Molecular Weight:
215.04
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
134 °C/15 mmHg (lit.)
mp
31-33 °C (lit.)
density
1.508 g/mL at 25 °C (lit.)
SMILES string
BrCC(=O)Oc1ccccc1
InChI
1S/C8H7BrO2/c9-6-8(10)11-7-4-2-1-3-5-7/h1-5H,6H2
InChI key
UEWYUCGVQMZMGY-UHFFFAOYSA-N
General description
Phenyl bromoacetate is an aromatic ester.
Application
Phenyl bromoacetate may be employed as alkylation reagent in the preparation of 2-(phenoxycarbonyl)methyl triazoles. It may be used in the synthesis of the A-ring of cylindrospermopsin. It may be used in the synthesis of the following 4-thiazolidinones:
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-3-methyl-1,3-thiazolidin-4-one
- 3-ethyl-2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene] hydrazinyl}-3-phenyl-1,3-thiazolidin-4-one
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Robert M Williams et al.
ACS symposium series. American Chemical Society, 1009, 420-442 (2010-06-22)
We report the application of diphenyloxazinone glycinate chiral templates to asymmetric syntheses of cylindrospermospin, 7-epi-cylindrospermopsin, 7-deoxycylindrospermopsin, and spirotryprostatins A and B. Synthetic studies toward quinine, nakadomarin A, and palau'amine using these templates are also described.
Duen-Ren Hou et al.
Bioorganic & medicinal chemistry letters, 19(3), 1022-1025 (2008-12-20)
This letter reports the new entry of novel 1,2,3-triazole derivatives as CB1 receptor antagonists. The design, synthesis and biological evaluation of N1 and N2 substituted 1,2,3-trizoles are described. The N2 substituted, symmetrical 1,2,3-triazoles are more potent ligands than the unsymmetrical
Synthesis and Antimicrobial Activity Evaluation of Novel 4-Thiazolidinones Containing a Pyrone Moiety.
Nechak R, et al.
Synthetic Communications, 1-11 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)