All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6OS
CAS Number:
Molecular Weight:
114.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.564 (lit.)
bp
86-88 °C/10 mmHg (lit.)
density
1.211 g/mL at 25 °C (lit.)
functional group
hydroxyl
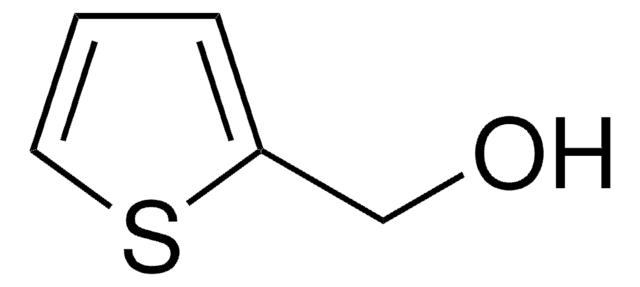
SMILES string
OCc1ccsc1
InChI
1S/C5H6OS/c6-3-5-1-2-7-4-5/h1-2,4,6H,3H2
InChI key
BOWIFWCBNWWZOG-UHFFFAOYSA-N
General description
Comparision of electrochemical polymerization properties of 3-thiophenemethanol and 3-methylthiophene has been reported.
Application
3-Thiophenemethanol was used in the preparation of 3-substituted thiophene conducting copolymers which has potential applications in electrochromic displays. It was also used in the synthesis of 4-(thiophene-3-ylmethoxy)phthalonitrile.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
213.8 °F - closed cup
Flash Point(C)
101 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A comparative study of the electrochemistry of 3-thiophenemethanol and 3-methylthiophene.
Pohjakallio M and Sundholm G.
Synthetic Metals, 55(2), 1590-1595 (1993)
Synthesis, molecular conformation, vibrational, electronic transition, and chemical shift assignments of 4-(thiophene-3-ylmethoxy) phthalonitrile: a combined experimental and theoretical analysis.
Coruh A, et al.
Structural Chemistry, 22(1), 45-56 (2011)
Electrochemical polymerization and characterization of new copolymers of 3-substituted thiophenes.
Alves MRA, et al.
Synthetic Metals, 160(1), 22-27 (2010)
Yuwei Hao et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 19(16), 2046-2051 (2018-03-25)
Highly efficient cell capture and release with low background are urgently required for early diagnosis of diseases such as cancer. Herein, we report an electrochemical responsive superhydrophilic surface exhibiting specific cell capture and release with high yields and extremely low
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service