300098
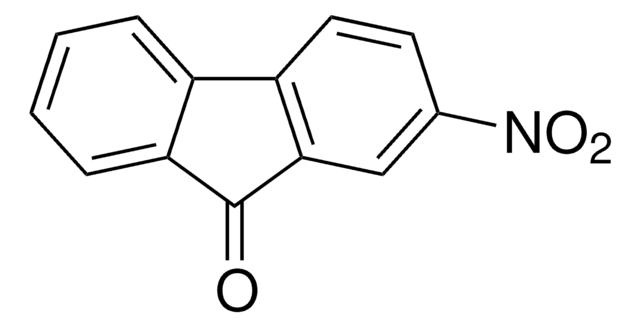
2,7-Dibromo-9-fluorenone
96%
Synonym(s):
2,7-Dibromo-9H-fluoren-9-one, 2,7-Dibromofluorenone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C13H6Br2O
CAS Number:
Molecular Weight:
337.99
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
203-205 °C (lit.)
functional group
bromo
ketone
SMILES string
Brc1ccc2-c3ccc(Br)cc3C(=O)c2c1
InChI
1S/C13H6Br2O/c14-7-1-3-9-10-4-2-8(15)6-12(10)13(16)11(9)5-7/h1-6H
InChI key
CWGRCRZFJOXQFV-UHFFFAOYSA-N
Related Categories
Application
2,7-Dibromo-9-fluorenone was used in preparation of :
- 2,7-poly(spiro[4′,4′-dioctyl-2′,6′-dioxocyclohexane-1′,9-fluorene]), precursor polymer for the synthesis of 2,7-poly(9-fluorenone)
- 2,7-dibromo-9-(2-methylpyridin-5-yl)fluoren-9-ol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A precursor route to 2, 7-poly (9-fluorenone).
Uckert F, et al.
Macromolecules, 32(14), 4519-4524 (1999)
Efficient White-Electrophosphorescent Devices Based on a Single Polyfluorene Copolymer.
Wu F-I, et al.
Advances in Functional Materials, 17(7), 1085-1092 (2007)
Guang-Wei Zhang et al.
International journal of molecular sciences, 14(11), 22368-22379 (2013-11-16)
Supramolecular luminescence stems from non-covalent exciton behaviors of active π-segments in supramolecular entities or aggregates via intermolecular forces. Herein, a π-conjugated oligofluorenol, containing self-complementary double hydrogen bonds, was synthesized using Suzuki coupling as a supramolecular semiconductor. Terfluorenol-based random supramolecular polymers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service