All Photos(1)
About This Item
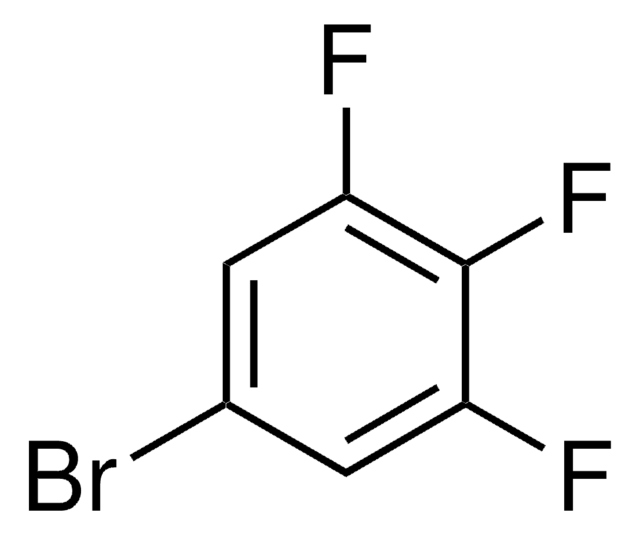
Linear Formula:
BrC6H2F3
CAS Number:
Molecular Weight:
210.98
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.485 (lit.)
bp
144 °C (lit.)
mp
−19 °C (lit.)
solubility
water: insoluble
density
1.802 g/mL at 25 °C (lit.)
functional group
bromo
fluoro
SMILES string
Fc1cc(F)c(Br)cc1F
InChI
1S/C6H2BrF3/c7-3-1-5(9)6(10)2-4(3)8/h1-2H
InChI key
DVTULTINXNWGJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Bromo-2,4,5-trifluorobenzene undergoes Br-Mg-exchange reaction with i-PrMgBr in THF to yield organomagnesium compound.
Application
1-Bromo-2,4,5-trifluorobenzene has been used in the synthesis of:
- 3-ethenyl, 3-ethynyl, 3-aryl and 3-cyclopropyl-2,4,5-trifluorobenzoic acids
- 3-bromo-2,5,6-trifluorobenzoic acid and 2,4,5-trifluorobenzoic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
132.8 °F - closed cup
Flash Point(C)
56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3-Ethenyl, 3-ethynyl, 3-aryl, and 3-cyclopropyl-2, 4, 5-trifluorobenzoic acids: Useful intermediate in the synthesis of quinolone antibacterials.
Turner WR and Suto MJ.
Tetrahedron Letters, 34(2), 281-284 (1993)
Bromine-magnesium-exchange as a general tool for the preparation of polyfunctional aryl and heteroaryl magnesium-reagents.
Abarbri M, et al.
Tetrahedron Letters, 40(42), 281-284 (1999)
A dramatic solvent effect during aromatic halogen-metal exchanges. Different products from lithiation of polyfluorobromobenzenes in ether and tetrahydrofuran.
Bridges AJ, et al.
The Journal of Organic Chemistry, 55(2), 773-775 (1990)
Chan Seok Oh et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(2), 642-648 (2018-10-20)
Blue thermally activated delayed fluorescent (TADF) devices with short excited-state lifetime, high reverse intersystem crossing rate, and low-efficiency roll-off were developed by managing the molecular structure of donor-acceptor-type blue emitters. Three isomers of blue TADF emitters with a diphenyltriazine acceptor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service