220868
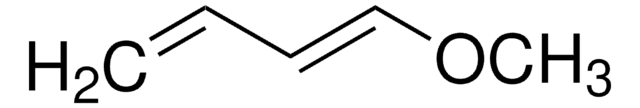
1-Acetoxy-1,3-butadiene
mixture of cis and trans
Synonym(s):
1,3-Butadienyl acetate
About This Item
Recommended Products
vapor pressure
40 mmHg ( 60 °C)
form
liquid
contains
0.1% p-tert-butylcatechol as stabilizer
refractive index
n20/D 1.469 (lit.)
bp
60-61 °C/40 mmHg (lit.)
density
0.945 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=O)O\C=C\C=C
InChI
1S/C6H8O2/c1-3-4-5-8-6(2)7/h3-5H,1H2,2H3/b5-4+
InChI key
NMQQBXHZBNUXGJ-SNAWJCMRSA-N
General description
Application
- Diels-Alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone.
- Diels-Alder reaction with diethyl ketovinylphosphonate, with and without Lewis acid assistance.
- Diels-Alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid.
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
91.4 °F - closed cup
Flash Point(C)
33 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service