214930
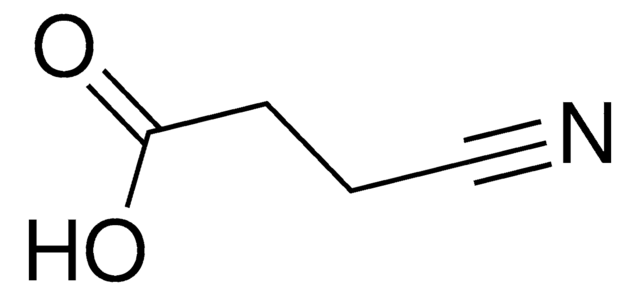
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid hydrochloride
96%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H11NO2 · HCl
CAS Number:
Molecular Weight:
213.66
Beilstein:
3723332
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
>300 °C (lit.)
SMILES string
Cl[H].OC(=O)C1Cc2ccccc2CN1
InChI
1S/C10H11NO2.ClH/c12-10(13)9-5-7-3-1-2-4-8(7)6-11-9;/h1-4,9,11H,5-6H2,(H,12,13);1H
InChI key
FXHCFPUEIDRTMR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1,2,3,4-Tetrahydro-3-isoquinolinecarboxylic acid was used in the synthesis of 10,10a-dihydroimidazo-[1,5-b]isoquinoline-1,3(2H,5H)-diones, inhibitor of inflammation, apoprotein B-100 biosynthesis and matrix-degrading metalloprotienase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alan R Katritzky et al.
The Journal of organic chemistry, 67(23), 8224-8229 (2002-11-09)
Condensations of chiral diamines 11a-c with benzotriazole and formaldehyde gave benzotriazolyl intermediates 12a-c; similar condensations of alpha-amino-amides 10a-c with benzotriazole and paraformaldehyde gave 14a-c. Subsequent treatment of 12a-c and 14a-c with AlCl(3) led to enantiopure tricyclic 1,2,3,5,10,10a-hexahydroimidazo[1,5-b]isoquinolines 1a-c and 2,3,10,10a-tetrahydroimidazo[1,5-b]isoquinolin-1(5H)-ones
P A Temussi et al.
Biochemical and biophysical research communications, 198(3), 933-939 (1994-02-15)
The surprising change of selectivity induced by the change of chirality in peptides containing the tetrahydro-3-isoquinoline carboxylic acid (Tic) in second position, interpreted as a conformational preference induced on the Tyr-Xaa-Phe domain, can instead be attributed to the Tyr-Tic message
Qian-Yu Zhao et al.
Protein and peptide letters, 12(4), 323-326 (2005-05-24)
[Tic(4)]EM1 and [Tic(4)]EM2, new endomorphins (EMs) analogues, caused relaxation of rat aorta rings precontracted with phenylphrine in a concentration-dependent manner and were 240- to 370-fold more potent than EMs. This effect was inhibited by endothelium removal or by incubation with
Bradford H Hirth et al.
Bioorganic & medicinal chemistry letters, 15(8), 2087-2091 (2005-04-06)
A series of 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid diamides that increase chloride transport in cells expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) protein has been identified from our compound library. Analoging efforts and the resulting structure-activity relationships uncovered are detailed. Compound potency
Li Li et al.
Nanomedicine : nanotechnology, biology, and medicine, 8(7), 1216-1222 (2012-01-18)
The modification of 3S-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (THIQA) with β-cyclodextrin (β-CD) provides an oral antithrombotic agent, 6-(3'S-isoquinoline-3'-carboxylaminoethylamino)-6-deoxy-β-CD (THIQA-β-CD). In aqueous solution THIQA-β-CD undergoes intermolecular inclusion complexation and forms pH-dependent nanostructures. The morphological feature of THIQA-β-CD is a nanocloud consisting of numerous particles
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![1,3-Bis[4-(dimethylamino)phenyl]-2,4-dihydroxycyclobutenediylium dihydroxide, bis(inner salt) Dye content 90 %](/deepweb/assets/sigmaaldrich/product/structures/301/519/500149b3-198c-44cf-b952-7e91f54fc48e/640/500149b3-198c-44cf-b952-7e91f54fc48e.png)



![2,4-Bis[4-(N,N-diphenylamino)-2,6-dihydroxyphenyl]squaraine 98%](/deepweb/assets/sigmaaldrich/product/structures/303/054/d8b9c845-3623-4f5a-8a30-ab6731034171/640/d8b9c845-3623-4f5a-8a30-ab6731034171.png)