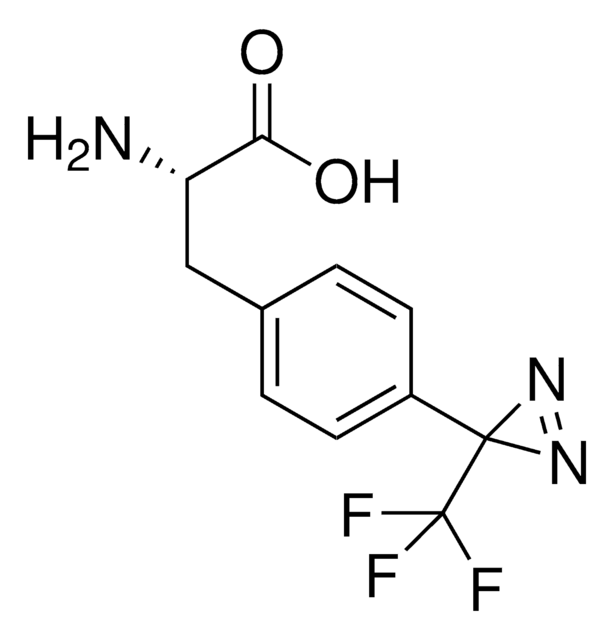
907294
Fmoc-L-Photo-Phe-OH
≥95%
Synonym(s):
(S)-2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-3-(4-(3-(trifluoromethyl)-3H-diazirin-3-yl)phenyl)propanoic acid, N-α-(9-Fluorenylmethyloxycarbonyl)-4-(trifluoromethyldiazirin)-L-phenylalanine, Diazirine amino acid, Fmoc-Tdf-OH, Photo-Phe, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Recommended Products
Assay
≥95%
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
−20°C
Application
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Mode of Action of cGMP-dependent Protein Kinase-specific Inhibitors Probed by Photoaffinity Cross-linking Mass Spectrometry
Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation
Simple and Versatile Method for Tagging Phenyldiazirine Photophores
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service