All Photos(2)
About This Item
Empirical Formula (Hill Notation):
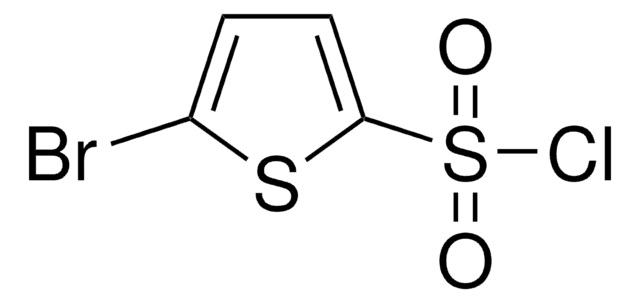
C4H2Cl2O2S2
CAS Number:
Molecular Weight:
217.09
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.586 (lit.)
bp
112-117 °C (lit.)
density
1.623 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Clc1ccc(s1)S(Cl)(=O)=O
InChI
1S/C4H2Cl2O2S2/c5-3-1-2-4(9-3)10(6,7)8/h1-2H
InChI key
SORSTNOXGOXWAO-UHFFFAOYSA-N
General description
5-Chlorothiophene-2-sulfonyl chloride can be prepared from 2-chlorothiophene by reacting with chlorosulfonic acid in the presence of phosphorus pentachloride.
Application
5-Chlorothiophene-2-sulfonyl chloride may be used in the preparation of:
- (S)-N-(5-chlorothiophene-2-sulfonyl)-β,β-diethylalaninol
- N-(3-(1H-pyrazol-1-yl)pyridin-2-yl)-5-chlorothiophene-2-sulfonamide
- 5-chloro-4-nitrothiophene-2-sulfonyl chloride
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A facile stereoselective total synthesis of (S)-N-(5-chlorothiophene-2-sulfonyl)-?,?-diethylalaninol.
Reddy BN and Sing RP.
Der Pharma Chemica, 7(1), 201-205 (2015)
C A Hunt et al.
Journal of medicinal chemistry, 37(2), 240-247 (1994-01-21)
3-Aminoalkyl derivatives of thieno[2,3-b][1,4]thiazine-6-sulfonamide were prepared for evaluation as topically active ocular hypotensive agents. The compounds described were found to be excellent in vitro inhibitors of carbonic anhydrase II and in vivo to lower intraocular pressure in three rabbit models
Afjal H Miah et al.
Organic & biomolecular chemistry, 12(11), 1779-1792 (2014-02-12)
A knowledge-based library of aryl 2,3-dichlorophenylsulfonamides was synthesised and screened as human CCR4 antagonists, in order to identify a suitable hit for the start of a lead-optimisation programme. X-ray diffraction studies were used to identify the pyrazole ring as a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service