361747
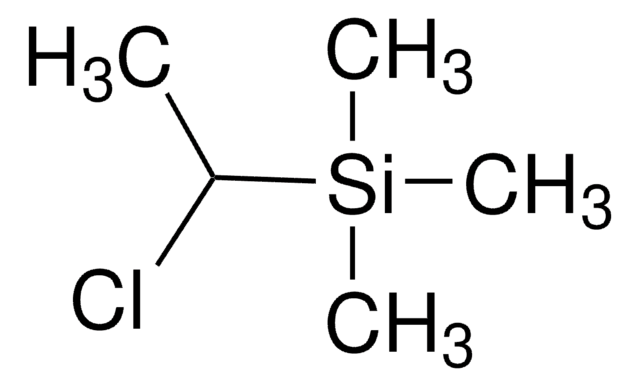
Chlorobis(trimethylsilyl)methane
97%
Synonym(s):
Bis(trimethylsilyl)chloromethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
[(CH3)3Si]2CHCl
CAS Number:
Molecular Weight:
194.85
Beilstein:
1736681
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.449 (lit.)
bp
57-60 °C/15 mmHg (lit.)
density
0.892 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
C[Si](C)(C)C(Cl)[Si](C)(C)C
InChI
1S/C7H19ClSi2/c1-9(2,3)7(8)10(4,5)6/h7H,1-6H3
InChI key
XNJGZHVYPBNLEB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Chlorobis(trimethylsilyl)methane can be used as a reagent for the preparation of:
- Para-bis(trimethylsilyl)ethylstyrene (PBTES) monomer, which is used to synthesize corresponding network polymer of styrene.
- Bis(trimethylsilyl)methyl magnesium chloride (Grignard reagent), which is used in the synthesis of bis(trimethylsilyl) allyl compounds by reacting with alkenyl bromide via the Kumada coupling reaction.
- N-[Bis(trimethylsilyl)methyl]heterocumulenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
118.4 °F - closed cup
Flash Point(C)
48 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and Reactivity of N-[Bis (trimethylsilyl) methyl] heterocumulenes
Barbaro G, et al.
The Journal of Organic Chemistry, 60(19), 6032-6039 (1995)
David R Williams et al.
Organic letters, 8(20), 4393-4396 (2006-09-22)
Allylation reagents, which possess geminal bis-trimethylsilyl substitution, are readily prepared from E- or Z-alkenyl bromides. The reactivity of 3,3-bis(trimethylsilyl)-2-methyl-1-propene (1) is described and predominantly provides ene reactions with aldehydes to give alcohol 2 in the presence of BF3.OEt2. Alternatively, Sakurai
Synthesis of some silyl mono-and polystyrenes with new properties
Assadi, MG and Hosseinzadeh, F
Designed Monomers and Polymers, 13(2), 181-191 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service