All Photos(1)
About This Item
Empirical Formula (Hill Notation):
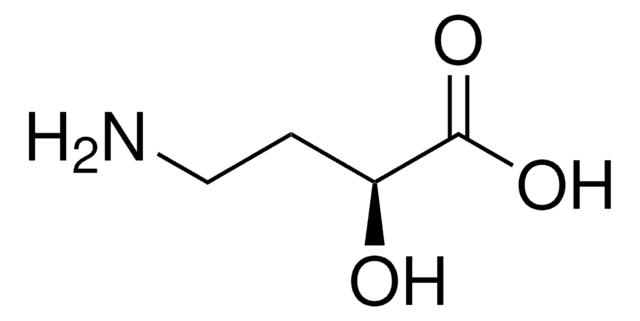
C4H9NO3
CAS Number:
Molecular Weight:
119.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D +20.0°, c = 1.7 in H2O
mp
207-212 °C
SMILES string
NC[C@@H](O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)/t3-/m0/s1
InChI key
YQGDEPYYFWUPGO-VKHMYHEASA-N
Related Categories
General description
(S)-(+)-4-Amino-3-hydroxybutyric acid [(S)-GABOB] can be prepared starting from ethyl (S)-4-chloro-3-hydroxybutyrate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Jordan et al.
Brain research, 268(1), 105-110 (1983-05-23)
Various doses of GABA from 0.25 to 5 mumol injected into the third ventricle decrease serum TSH rapidly. The same effect was observed with GABOB (10 mumol), the hydroxylated form of GABA. The inhibitory effect of both of these drugs
GABAergic control of anterior pituitary function in humans.
G B Melis et al.
Advances in biochemical psychopharmacology, 42, 219-242 (1986-01-01)
[Neurochemical aspects of the pharmacology of GABAergic substances].
K S Raevskiĭ
Farmakologiia i toksikologiia, 44(5), 517-529 (1981-09-01)
T W Stone
European journal of pharmacology, 128(1-2), 81-83 (1986-08-22)
Kynurenine and kynurenic acid are known to produce convulsions in rats and mice and it has been reported that kynurenine can displace GABA from its neuronal binding sites. The present study shows that neither kynurenine nor kynurenic acid are antagonist
T Hata et al.
Nihon yakurigaku zasshi. Folia pharmacologica Japonica, 91(3), 163-171 (1988-03-01)
SART-stressed (repeated cold-stressed) rats, experimental model animals for vagotonic-type dysautonomia, have been reported to show EEG with lower-amplitude fast waves during resting-arousal and higher-amplitude slow waves during slow-wave sleep compared to normal rats. In this report, the effects of certain
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service