183695
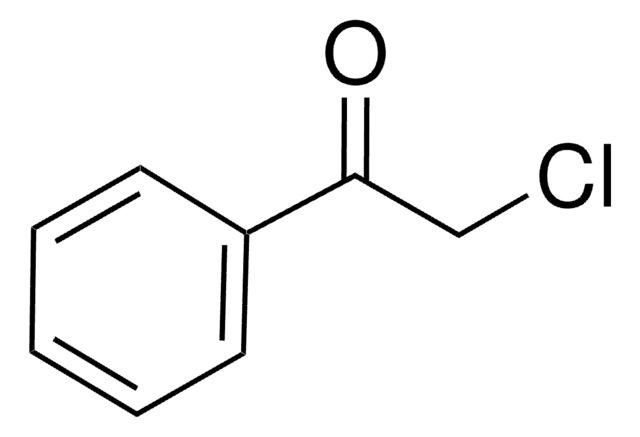
2′-Bromoacetophenone
99%
Synonym(s):
1-Acetyl-2-bromobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4COCH3
CAS Number:
Molecular Weight:
199.04
Beilstein:
1931534
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.568 (lit.)
density
1.476 g/mL at 25 °C (lit.)
functional group
bromo
ketone
SMILES string
CC(=O)c1ccccc1Br
InChI
1S/C8H7BrO/c1-6(10)7-4-2-3-5-8(7)9/h2-5H,1H3
InChI key
PIMNFNXBTGPCIL-UHFFFAOYSA-N
Related Categories
General description
2′-Bromoacetophenone (2-Bromoacetophenone) undergoes enantioselective addition reaction with phenylacetylene catalyzed by chiral camphorsulfonamide. It reacts with aliphatic primary amines in the presence of palladium catalyst to afford 3-methyleneisoindolin-1-ones.
Application
2′-Bromoacetophenone (2-Bromoacetophenone) was used in the synthesis of novel series of non-condensed 5,5-bicycles.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enantioselective alkynylation of aromatic ketones catalyzed by chiral camphorsulfonamide ligands.
Gui Lu et al.
Angewandte Chemie (International ed. in English), 42(41), 5057-5058 (2003-11-05)
Palladium-catalysed convenient synthesis of 3-methyleneisoindolin-1-ones.
Cho CS, et al.
Synthetic Communications, 32(!2), 1821-1827 (2002)
Non-Condensed Trifluoromethylated 5, 5-Bicycles: Synthesis of 2-[3-Alkyl (phenyl)-1H-pyrazol-1-yl]-4-phenyl-5-alkylthiazole and-4, 5, 6, 7-tetrahydrobenzothiazole Systems.
Bonacorso HG, et al.
Synthesis, 2002(08), 1079-1083 (3003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![2-phenylimidazo[1,2-a]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/281/247/6c2550a0-2f0c-4866-83d8-3c1fb039e165/640/6c2550a0-2f0c-4866-83d8-3c1fb039e165.png)