143685
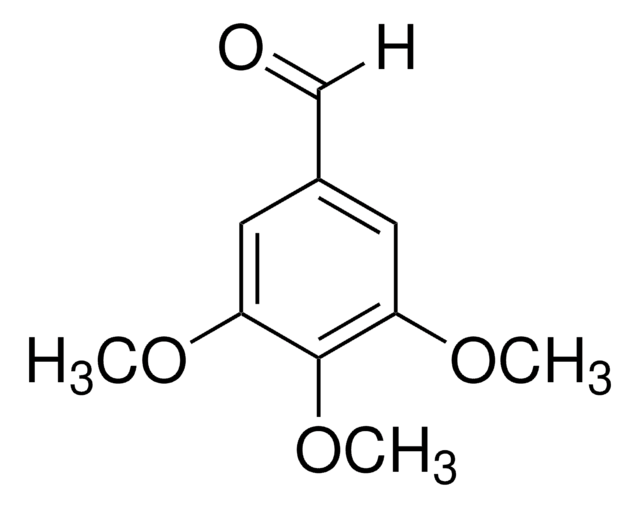
3-Hydroxy-4-methoxybenzaldehyde
99%
Synonym(s):
3-Hydroxyanisaldehyde, Isovanillin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1073021
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
179 °C/15 mmHg (lit.)
mp
113-115 °C (lit.)
SMILES string
[H]C(=O)c1ccc(OC)c(O)c1
InChI
1S/C8H8O3/c1-11-8-3-2-6(5-9)4-7(8)10/h2-5,10H,1H3
InChI key
JVTZFYYHCGSXJV-UHFFFAOYSA-N
General description
3-Hydroxy-4-methoxybenzaldehyde on condensation with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide yields Schiff-bases. It undergoes condensation reaction with1-azabicyclo[2.2.2]octan-3-one to give (Z)-2-(3-hydroxy-4-methoxybenzylidene)-1-azabicyclo[2.2.2]octan-3-one.
Application
3-Hydroxy-4-methoxybenzaldehyde was used as starting reagent during the two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 and glycitein synthesis.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>212.0 °F
Flash Point(C)
> 100 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Gaukroger et al.
The Journal of organic chemistry, 66(24), 8135-8138 (2001-11-28)
A high-yielding, two-step stereoselective synthesis of the anticancer drug (Z)-combretastatin A-4 (1) has been devised. The method uses the Perkin condensation of 3,4,5-trimethoxyphenylacetic acid and 3-hydroxy-4-methoxybenzaldehyde followed by decarboxylation of the cinnamic acid intermediate using copper and quinoline. The iodine-catalyzed
Caroline Lang'at-Thoruwa et al.
Journal of natural products, 66(1), 149-151 (2003-01-25)
4-Methoxyresorcinol (3) was synthesized as the precursor for glycitein (6) synthesis by the oxidation of 3-hydroxy-4-methoxybenzaldehyde (1) to the aryl formate with H2O2 and a catalytic amount of SeO2. Glycitein (6) was synthesized by cyclization of 2,4,4'-trihydroxy-5-methoxydeoxybenzoin (5) with N,N-dimethylformamide
Riyadh M Ahmed et al.
TheScientificWorldJournal, 2013, 754868-754868 (2013-09-13)
New monomeric cobalt and cadmium complexes with Schiff-bases, namely, N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]furan-2-carbohydrazide (L¹) and N'-[(E)-(3-hydroxy-4-methoxyphenyl)methylidene]thiophene-2-carbohydrazide (L²) are reported. Schiff-base ligands L¹ and L² were derived from condensation of 3-hydroxy-4-methoxybenzaldehyde (iso-vanillin) with furan-2-carboxylic acid hydrazide and thiophene-2-carboxylic acid hydrazide, respectively. Complexes of the
Vijayakumar N Sonar et al.
Acta crystallographica. Section C, Crystal structure communications, 59(Pt 11), o647-o649 (2003-11-08)
Crystals of the title compound, C(15)H(17)NO(3), were obtained from a condensation reaction of 3-hydroxy-4-methoxybenzaldehyde with 1-azabicyclo[2.2.2]octan-3-one and subsequent crystallization of the product from methanol. The title compound, containing a double bond that connects the azabicyclic ring system to the 3-hydroxy-4-methoxybenzylidene
Antonio Rescigno et al.
Drug testing and analysis, 3(3), 176-181 (2010-12-03)
A number of vanilloids have been tested as tyrosinase inhibitors using Ligand-Based Virtual Screening (LBVS) driven by QSAR (Quantitative Structure-Activity Relationship) models as the multi-agent classification system. A total of 81 models were used to screen this family. Then, a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service