W515809
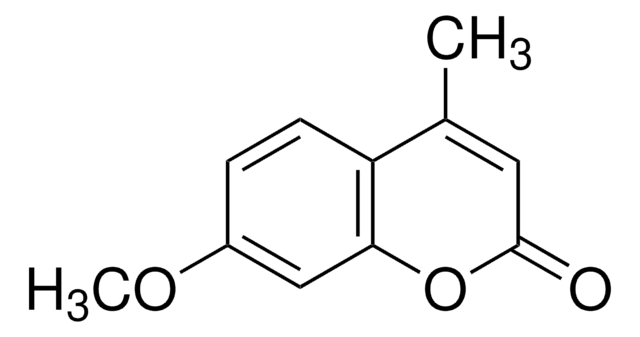
7-Methoxycoumarin
≥98%
Synonym(s):
Herniarin, Methyl umbelliferyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H8O3
CAS Number:
Molecular Weight:
176.17
Beilstein:
141728
EC Number:
MDL number:
UNSPSC Code:
12164502
PubChem Substance ID:
NACRES:
NA.21
Organoleptic:
balsam
biological source:
synthetic
food allergen:
no known allergens
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98%
mp
115-121 °C
117-121 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
balsam
SMILES string
COc1ccc2C=CC(=O)Oc2c1
InChI
1S/C10H8O3/c1-12-8-4-2-7-3-5-10(11)13-9(7)6-8/h2-6H,1H3
InChI key
LIIALPBMIOVAHH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
7-Methoxycoumarin is one of the main volatile odorant found in key lime essential oil and tarragon leaves.
Disclaimer
For R&D or non-EU Food use. Not for retail sale.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Impact of estragole and other odorants on the flavour of anise and tarragon
Zeller A and Rychlik M
Flavour and Fragrance Journal, 22(2), 105-113 (2007)
Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle).
Chisholm MG, et al.
Flavour and Fragrance Journal, 18(2), 106-115 (2003)
Miroslav Repcák et al.
Plant cell reports, 28(7), 1137-1143 (2009-05-12)
Chamomile (Matricaria chamomilla) in the above-ground organs synthesizes and accumulates (Z)- and (E)-2-beta-D: -glucopyranosyloxy-4-methoxy cinnamic acids (GMCA), the precursors of phytoanticipin herniarin (7-methoxycoumarin). The diurnal rhythmicity of the sum of GMCA (maximum before daybreak) and herniarin (acrophase at 10 h
Evy Paulsen et al.
Contact dermatitis, 62(6), 338-342 (2010-06-19)
Although German chamomile (Chamomilla recutita) is considered a weak sensitizer, recent studies have shown several possible non-sesquiterpene lactone allergens in tea (infusions) from the plant. The aim of this study was to report the results of patch testing with herniarin
Prince Firdoos Iqbal et al.
European journal of medicinal chemistry, 44(5), 2252-2259 (2008-08-05)
In continuation of our search for potential antiamoebic agents from folklore Indian medicinal plants, we found that the benzene and ethyl acetate extracts from the root bark of Adina cordifolia exhibited strong antiamoebic activity with IC(50) values of 2.92 and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service