All Photos(2)
About This Item
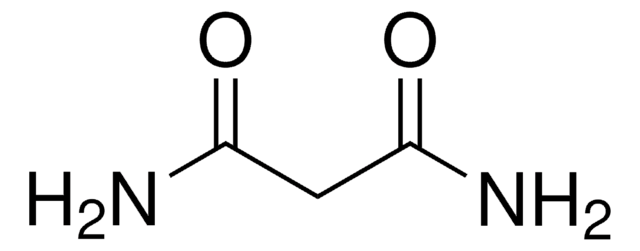
Linear Formula:
NH2COCONH2
CAS Number:
Molecular Weight:
88.07
Beilstein:
1743262
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
NC(=O)C(N)=O
InChI
1S/C2H4N2O2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
InChI key
YIKSCQDJHCMVMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Oxamide can be used:
- As a precursor for the synthesis of ligands such as bis(benzimidazole) and Schiff base ligands formed by condensation with furfural.
- Carbon nitride (g-C3N4) nanotubes by self-assembly polymerization with urea.
- As a bridging ligand for the synthesis of binuclear IrIII complex [Ir2(μ2-oxamidato-N,N′,O,O′)(ptpy)4], ptpy = 2-(p-tolyl)pyridinato.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bis (benzimidazole) as supramolecular building block in manganese (IV) chemistry
Samol'ova E, et al.
Journal of Molecular Structure, 1176, 366-375 (2019)
E Armelin et al.
The Journal of organic chemistry, 66(24), 8076-8085 (2001-11-28)
The conformational properties of the oxalamide group and crystal structure of several polyoxalamides have been investigated by computational methods. First, a detailed quantum mechanical study of the conformational preferences of N,N'-dimethyloxalamide is reported. Results, which were obtained at the MP2/6-31G(d)
Li-Na Zhu et al.
Inorganic chemistry, 46(4), 1297-1304 (2007-01-25)
Four heteronuclear complexes Mn(CuL)2(SCN)2 (1), {[Mn(CuL)2(mu-dca)2].2H2O}n (2), Zn(CuL)2(SCN)2 (3), and [Fe(CuL)(N3)2]2 (4) incorporating macrocyclic oxamide ligands have been synthesized and structurally characterized. L is the dianion of diethyl 5,6,7,8,15,16-hexahydro-6,7-dioxodibenzo[1,4,8,11]-tetraazacyclotetradecine-13,18-dicarboxylate, and dca is the dicyanamide. The structure of 1 or 3
Sergey P Gavrish et al.
Dalton transactions (Cambridge, England : 2003), (41)(41), 4708-4714 (2007-10-18)
A comparison of the molecular structure of related nickel(II) complexes of the open-chain and 13-membered macrocyclic oxamide-derived ligands NiL(1).4H2O and NiL(2).3H2O revealed that the formation of an additional 6-membered chelate ring in the complex results in rather small changes in
Yun Fei Long et al.
Analytica chimica acta, 624(1), 128-132 (2008-08-19)
Light scattering (LS) signals have been applied for analytical detections, but the selectivity is poor. In order to improve the selectivity, pre-separation or new machines are generally considered. Differing from these methods, we synthesized a highly selective oxamide ligand, N',N'-bis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service