All Photos(2)
About This Item
Empirical Formula (Hill Notation):
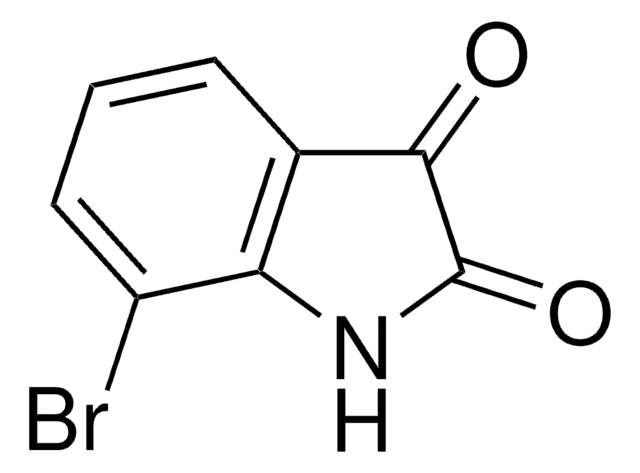
C8H4BrNO2
CAS Number:
Molecular Weight:
226.03
Beilstein:
383760
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
impurities
<10% isatin
mp
247-252 °C (lit.)
functional group
bromo
ketone
SMILES string
Brc1ccc2NC(=O)C(=O)c2c1
InChI
1S/C8H4BrNO2/c9-4-1-2-6-5(3-4)7(11)8(12)10-6/h1-3H,(H,10,11,12)
InChI key
MBVCESWADCIXJN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Bromoisatin is a 5-haloisatin. One of the methods reported for its synthesis is by reacting N-halosaccharins with isatin in the presence of SiO2. Its inotropic activity has been studied on rhythmically stimulated papillary muscles of guinea pigs. It is reported to exhibit analgesic and sedative properties at a dose of 0.2g/kg in mice.
Application
5-Bromoisatin may be used in the synthesis of the following:
- N-derivatives of 5-bromoisatin
- N-substituted pyrroles
- linear polyaryleneoxindoles
- 5-bromodioxindole
- cinchoninic acid derivatives
- 3-hydroxyoxindole
- S-benzyldithiocarbazate Schiff Bases
- 5-bromooxindole
- Morita-Baylis-Hillman adducts of isatin derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and spectral data for cinchoninic acids.
Sarkis GY.
Journal of Chemical and Engineering Data, 17(3), 388-391 (1972)
SiO2 mediated reaction of isatin with N-halosaccharins: A regiospecific preparation of 5-haloisatins.
de Souza SPL, et al.
Heterocyclic Communications, 9(1), 31-34 (2003)
Electro-organic synthesis of nanosized particles of 3-hydroxy-3-(1H-indol-3-yl) indolin-2-one derivatives.
Makarem S, et al.
Monatshefte fur Chemie / Chemical Monthly, 148(8), 1157-1160 (2012)
Quick and efficient synthesis of Morita-Baylis-Hillman adducts of isatin derivatives.
Rad-Moghadam K and Youseftabar-Miri L.
ARKIVOC (Gainesville, FL, United States), 11, 43-50 (2011)
Superelectrophiles in aromatic polymer chemistry.
Colquhoun HM, et al.
Macromolecules, 34(4), 1122-1124 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service