412201
L-Serine methyl ester hydrochloride
98%, for peptide synthesis
Synonym(s):
L-Serine methyl ester·HCl
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
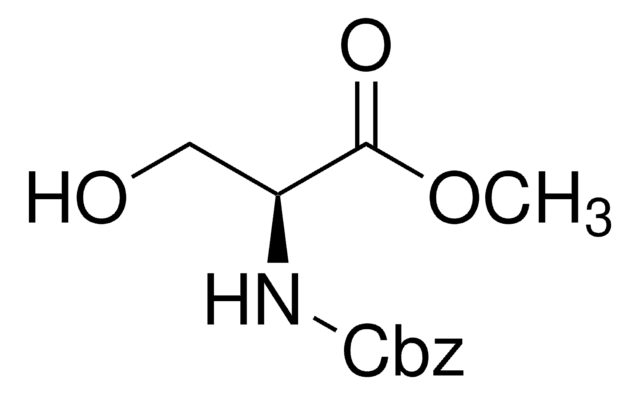
HOCH2CH(NH2)COOCH3 · HCl
CAS Number:
Molecular Weight:
155.58
Beilstein:
3559591
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
L-Serine methyl ester hydrochloride, 98%
Quality Level
Assay
98%
form
powder
optical activity
[α]20/D +3.4°, c = 4 in methanol
reaction suitability
reaction type: solution phase peptide synthesis
mp
163 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Cl.COC(=O)[C@@H](N)CO
InChI
1S/C4H9NO3.ClH/c1-8-4(7)3(5)2-6;/h3,6H,2,5H2,1H3;1H/t3-;/m0./s1
InChI key
NDBQJIBNNUJNHA-DFWYDOINSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carlos Aydillo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(17), 4840-4848 (2007-03-17)
A new chiral serine equivalent and its enantiomer have been synthesized from (S)- and (R)-N-Boc-serine methyl esters (Boc: tert-butyloxycarbonyl). The use of these compounds as chiral building blocks has been demonstrated in the synthesis of alpha-alkyl alpha-amino acids by diastereoselective
Yu Harayama et al.
Chemical communications (Cambridge, England), (13)(13), 1764-1766 (2005-03-26)
The use of hypervalent iodine(III) reagents allowed us to develop the novel and efficient direct synthesis of N,O-acetal compounds via the oxidative fragmentation reaction of alpha-amino acids or alpha-amino alcohols; furthermore, we succeeded in developing an improved synthesis of the
Ernest Mordret et al.
Molecular cell, 75(3), 427-441 (2019-07-30)
The translation machinery and the genes it decodes co-evolved to achieve production throughput and accuracy. Nonetheless, translation errors are frequent, and they affect physiology and protein evolution. Mapping translation errors in proteomes and understanding their causes is hindered by lack
Dragana Ahel et al.
FEBS letters, 579(20), 4344-4348 (2005-08-02)
Seryl-tRNA synthetases (SerRSs) fall into two distinct evolutionary groups of enzymes, bacterial and methanogenic. These two types of SerRSs display only minimal sequence similarity, primarily within the class II conserved motifs, and possess distinct modes of tRNA(Ser) recognition. In order
Acylated serine derivatives: a unique class of arthropod pheromones of the Australian redback spider, Latrodectus hasselti.
Elena Jerhot et al.
Angewandte Chemie (International ed. in English), 49(11), 2037-2040 (2010-02-11)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service