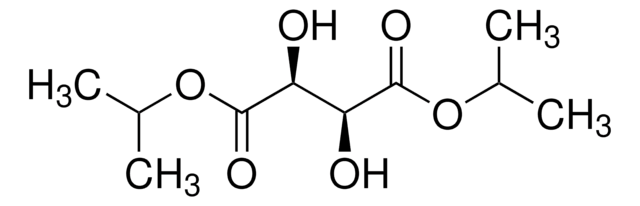
213969
(−)-Diethyl D-tartrate
≥99%
Synonym(s):
D-(−)-Tartaric acid diethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HO2CCCH(OH)CH(OH)CO2H
CAS Number:
Molecular Weight:
206.19
Beilstein:
1727143
EC Number:
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
optical activity
[α]23/D −8.5°, neat
optical purity
ee: ≥99% (GLC)
refractive index
n20/D 1.446 (lit.)
bp
162 °C/19 mmHg (lit.)
density
1.205 g/mL at 20 °C (lit.)
functional group
ester
hydroxyl
SMILES string
CCOC(=O)[C@@H](O)[C@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m0/s1
InChI key
YSAVZVORKRDODB-WDSKDSINSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(-)-Diethyl D-tartrate can be used as a starting material in the synthesis of bioactive compounds such as (-)-kumausallene, syringolide 2 and (+)-exo-brevicomin.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
200.1 °F - closed cup
Flash Point(C)
93.4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and absolute configuration of syringolide 2, an elicitor from Pseudomonas syringae pv. tomato.
Kuwahara S, et al.
Tetrahedron Letters, 36(18), 3201-3202 (1995)
A new expeditious synthesis of (+)-exo-brevicomin via efficient C? C bond formation of triflates.
Kotsuki H, et al.
Tetrahedron Letters, 30(30), 3999-4000 (1989)
Radical cyclization of ?-alkoxyacrylates: A formal synthesis of (-)-kumausallene.
Lee E, et al.
Tetrahedron Letters, 39(3), 317-318 (1998)
Jiang Weng et al.
The Journal of organic chemistry, 75(9), 3125-3128 (2010-04-17)
A short and practical synthesis of oseltamivir was accomplished in 11 steps from inexpensive and abundant diethyl D-tartrate starting material. This azide-free route featured an asymmetric aza-Henry reaction and a domino nitro-Michael/Horner-Wadsworth-Emmons (HWE) reaction as the key steps to construct
Total synthesis of the light-harvesting carotenoid peridinin.
Thomas Olpp et al.
Angewandte Chemie (International ed. in English), 45(24), 4023-4027 (2006-05-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service