115819
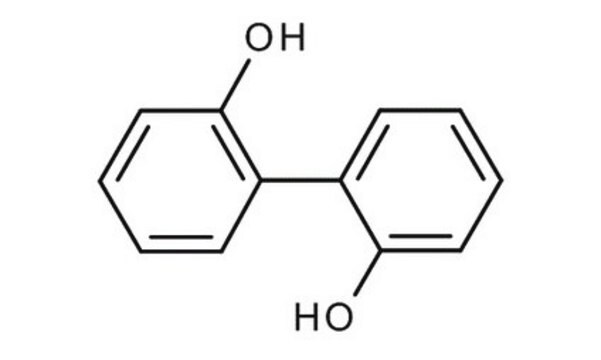
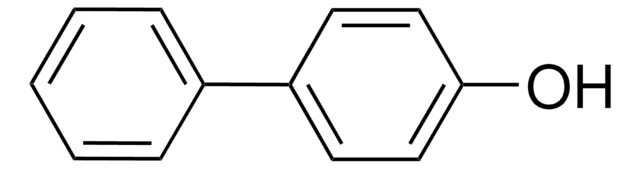
2,2′-Biphenol
99%
Synonym(s):
2,2′-Biphenyldiol, 2,2′-Dihydroxybiphenyl, 2,2′-Diphenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4C6H4OH
CAS Number:
Molecular Weight:
186.21
Beilstein:
1638363
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
solid
bp
315 °C (lit.)
mp
108-110 °C (lit.)
SMILES string
Oc1ccccc1-c2ccccc2O
InChI
1S/C12H10O2/c13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-8,13-14H
InChI key
IMHDGJOMLMDPJN-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
309.2 °F - closed cup - (External MSDS)
Flash Point(C)
154 °C - closed cup - (External MSDS)
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Atsushi Kuwahara et al.
The Journal of organic chemistry, 70(2), 413-419 (2005-01-18)
The double N-arylation of primary amines with 2,2'-biphenylylene ditriflates was investigated for the synthesis of multisubstituted carbazoles. Palladium complexes supported by 2-dicyclohexylphosphino-2'-methylbiphenyl or Xantphos [4,5-bis(diphenylphosphino)-9,9-dimethylxanthene] were found to be efficient catalysts for the reaction. The catalysts allow the use of
Zengqi Xie et al.
Organic letters, 12(14), 3204-3207 (2010-06-22)
Facile nucleophilic substitution of two chlorine atoms by 2,2'-biphenol at one of the two bay areas (1,12- and 6,7-positions) of core-tetrachlorinated perylene bisimide afforded a novel, completely desymmetrized perylene bisimide building block, which could be further functionalized by substitution of
H P Kohler et al.
Journal of bacteriology, 175(6), 1621-1628 (1993-03-01)
Cells of Pseudomonas sp. strain HBP1 grown on 2-hydroxy- or 2,2'-dihydroxybiphenyl contain NADH-dependent monooxygenase activity that hydroxylates 2,2'-dihydroxybiphenyl. The product of this reaction was identified as 2,2',3-trihydroxybiphenyl by 1H nuclear magnetic resonance and mass spectrometry. Furthermore, the monooxygenase activity also
T Kuhnigk et al.
Journal of basic microbiology, 37(3), 205-211 (1997-01-01)
The capability of the intestinal flora from the gut of xylophagous termites of degrading lignin model compounds was investigated. Different dimeric lignin model compounds-degrading bacteria were obtained from the hindgut flora of Mastotermes darwiniensis Froggatt, Reticulitermes santonensis Feytaud, Nasutitermes nigriceps
W A Prütz et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 44(2), 183-196 (1983-08-01)
Phenoxyl radicals generated pulse radiolytically by the reaction of N.3 with Gly-Tyr decay biomolecularly (2k = 4.7 X 10(8)M-1 s-1) with efficient formation of 2,2'-dimers, which enolize rapidly (k = 2.7 X 10(4) s-1) to produce the 2,2'-biphenolic product. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service