SBR00036
Rhodamine B labeled Polymyxin B Ready Made Solution
For fluorescent microbial imaging, 1.1 mg/mL in water
Synonym(s):
SRB-PMB
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352211
NACRES:
NA.47
Recommended Products
Related Categories
General description
Rhodamine B labeled polymyxin B (SRB-PMB) is a fluorescent derivative of Polymyxin B. Rhodamine B polymyxin B is an additional fluorescent derivative of Polymyxin B, along with Dansyl labeled polymyxin B (Product # SBR00029). Both products′ mode of action is in accordance with Polymyxin B activity. Fluorescent antibiotics are obtained by synthetic conjugation of an antibiotic to a fluorophore.
Application
Fluorescent antibiotics can be used for many applications including:
Additional possible applications for Rhodamine B labeled Polymyxin B:
- Antimicrobial resistance research.
- Bacterial visualization and imaging.
- Parent antibiotic mode of action research and new antibiotic discovery.
- Toxicity studies.
- Research of bacterial infections and tracking its uptake in vivo.
Additional possible applications for Rhodamine B labeled Polymyxin B:
- Evaluate LPS binding by FRET (fluorescence resonance energy transfer) studies to form a specific complex between fluorescein (FITC)-tagged LPS and Polymyxin B, indicating direct binding of the antibiotic to the LPS
- Can be applied as a fluorescent probe to study polymyxin mode of action and its pharmacokinetics
- to assess the mitochondrial function of polymyxin B in a kidney cell line (LLC-PK1), suggesting that it changes the mitochondrial membrane polarization.
Analysis Note
- Rhodamine B labeled polymyxin B Ready Made Solution is light sensitive.
- It is recommended to avoid freeze-thaw cycles of Rhodamine B labeled polymyxin B Ready-Made Solution
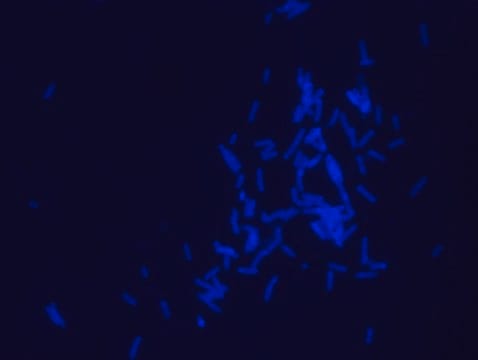
- Rhodamine B labeled polymyxin B Ready Made Solution (1.1mg/mL) can be diluted 1:100-200 in PBSX1 (Product# D8537) to achieve 0.55-1.1μg/mL final concentration for staining. The above concentration of Rhodamine B labeled polymyxin B was used for Escherichia coli staining (see image)
- Fluorescence Microscopy application: Rhodamine B labeled polymyxin B Ready Made Solution excitation (Ex) wavelength is 550-560nm resulting in emission (Em) range of 580-610nm (λmax=590-600nm)
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kinetics and Mechanism of the Recognition of Endotoxin by Polymyxin B
Thomas C J, et al.
Journal of the American Chemical Society, 120(48), 12428-12434 (1998)
P R Schindler et al.
Antimicrobial agents and chemotherapy, 8(1), 95-104 (1975-07-01)
Though the primary action of the cationic antibiotic polymyxin B is against the membrane of susceptible bacteria, severe morphological changes are detected in the cytoplasm. Using fluorescence microscopy and a mono-N-dansyl-polymyxin B derivative, we could demonstrate aggregations of the antibiotic
Vincent H Tam et al.
Antimicrobial agents and chemotherapy, 49(9), 3624-3630 (2005-08-30)
Despite limited data, polymyxin B (PB) is increasingly used clinically as the last therapeutic option for multidrug-resistant (MDR) gram-negative bacterial infections. We examined the in vitro pharmacodynamics of PB against four strains of Pseudomonas aeruginosa. Clonal relatedness of the strains
R E Hancock
Lancet (London, England), 349(9049), 418-422 (1997-02-08)
The era of the "classical antibiotic" may be over. The emergence of resistance has seen to that. Yet no truly novel class of antibacterial agent has come on the market in the past 30 years. Currently there is great interest
Zakuan Z Deris et al.
Bioconjugate chemistry, 25(4), 750-760 (2014-03-19)
The dry antibiotic development pipeline coupled with the emergence of multidrug resistant Gram-negative 'superbugs' has driven the revival of the polymyxin lipopeptide antibiotics. Polymyxin resistance implies a total lack of antibiotics for the treatment of life-threatening infections. The lack of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service