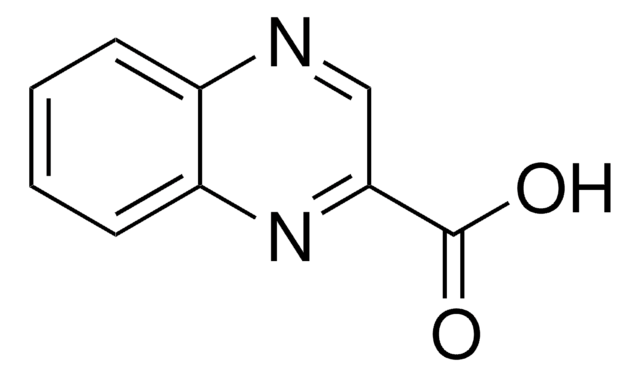
Q1603
Quinoxaline
≥98%
Synonym(s):
1,4-Benzodiazine, Benzo[a]pyrazine, Benzopyrazine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6N2
CAS Number:
Molecular Weight:
130.15
Beilstein:
109351
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
bp
220-223 °C (lit.)
mp
29-32 °C (lit.)
density
1.124 g/mL at 25 °C (lit.)
SMILES string
c1ccc2nccnc2c1
InChI
1S/C8H6N2/c1-2-4-8-7(3-1)9-5-6-10-8/h1-6H
InChI key
XSCHRSMBECNVNS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mark T Bilodeau et al.
Bioorganic & medicinal chemistry letters, 18(11), 3178-3182 (2008-05-16)
A series of naphthyridine and naphthyridinone allosteric dual inhibitors of Akt1 and 2 have been developed. These compounds have been optimized to have potent dual activity against the activated kinase as well as the activation of Akt in cells. One
Wei Zhang et al.
Journal of agricultural and food chemistry, 67(22), 6350-6358 (2019-05-16)
α-Dicarbonyls are reactive intermediates formed during Maillard reactions and carbohydrate degradation. The formation of seven α-dicarbonyls was characterized in solutions containing dairy related carbohydrates (galactose, glucose, lactose, and galacto-oligosaccharides (GOS)) during incubations at 40 and 50 °C with and without
Neslihan Göncüoğlu Taş et al.
Journal of agricultural and food chemistry, 67(1), 415-424 (2018-12-12)
This study investigated the effect of roasting (150 °C for 30 min) and storage (12 months at 4 °C, 25 °C, and 25 °C in vacuum package), conditions of which are generally applied in the industry and markets, on the
F Docobo-Pérez et al.
Antimicrobial agents and chemotherapy, 59(9), 5602-5610 (2015-07-01)
The aim of this study was to improve the understanding of the pharmacokinetic-pharmacodynamic relationships of fosfomycin against extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli strains that have different fosfomycin MICs. Our methods included the use of a hollow fiber infection model with
Ecem Berk et al.
Journal of agricultural and food chemistry, 67(17), 4923-4930 (2019-04-11)
This study investigated the formation of Maillard reaction products in sesame seeds under different roasting conditions. Sesame seeds were roasted at 150, 180, 200, and 220 °C for 10 min, and thermal process contaminants including 5-hydroxymethylfurfural, acrylamide, furan, and dicarbonyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service