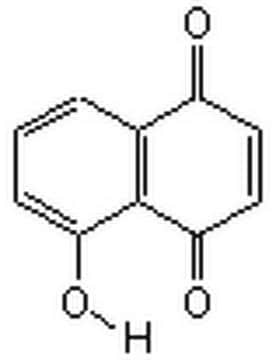
H47003
5-Hydroxy-1,4-naphthoquinone
97%
Synonym(s):
Juglone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H6O3
CAS Number:
Molecular Weight:
174.15
Beilstein:
1909764
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
161-163 °C (lit.)
SMILES string
Oc1cccc2C(=O)C=CC(=O)c12
InChI
1S/C10H6O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-5,12H
InChI key
KQPYUDDGWXQXHS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
5-Hydroxy-1,4-naphthoquinone (juglone) can be used as a starting material for the synthesis of:
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
- Trypanocidal drugs.
- Tacrine-naphthoquinone hybrids with potential application in the treatment of Alzheimer′s disease.
- Juglone-based electroactive polymer, poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-3-thioacetic acid-1,4-naphthoquinone), for the electrochemical detection of DNA hybridization.
- Ent-nocardione A, naturally-occurring tyrosine phosphatase inhibitor.
Juglone can also be used as a dienophile in the Diels–Alder reaction for the synthesis of variety of C-aryl glycosides.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-and 3-substituted 1, 4-naphthoquinone derivatives as subversive substrates of trypanothione reductase and lipoamide dehydrogenase from trypanosoma c ruzi: synthesis and correlation between redox cycling activities and in vitro cytotoxicity.
Salmon-Chemin L, et al.
Journal of Medicinal Chemistry, 44(4), 548-565 (2001)
Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-β aggregation and to exert anticholinesterase and antioxidant effects.
Nepovimova E, et al.
Journal of medicinal chemistry, 57(20), 8576-8589 (2014)
Formal Radical Cyclization onto Benzene Rings: A General Method and Its Use in the Synthesis of ent-Nocardione A.
Clive DLJ, et al.
The Journal of Organic Chemistry, 69(10), 3282-3293 (2004)
Study of the DNA hybridization transduction behavior of a quinone-containing electroactive polymer by cyclic voltammetry and electrochemical impedance spectroscopy.
Piro B, et al.
Journal of Electroanalytical Chemistry, 577(1), 155-165 (2005)
Kejie Wang et al.
Experimental gerontology, 112, 20-29 (2018-08-19)
Growing evidence shows that enhanced reactive oxygen species (ROS) production is an important contributor to obesity and its co-morbidities, but the functional link between ROS and obesity remains elusive. In this study we used the model animal Caenorhabditis elegans to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service