851450
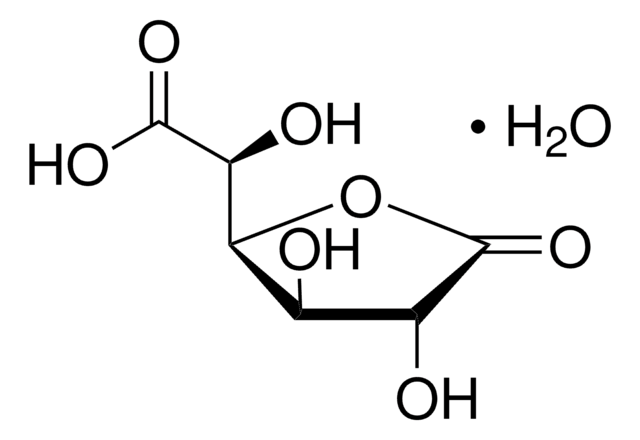
D-(+)-Glucuronic acid γ-lactone
≥99%
Synonym(s):
D-(+)-Glucurono-6,3-lactone, D-Glucurone, D-Glucurono-6,3-lactone, Glucuronolactone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H8O6
CAS Number:
Molecular Weight:
176.12
Beilstein:
83595
EC Number:
MDL number:
UNSPSC Code:
12352115
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
powder
optical activity
[α]24/D +18.8°, c = 8 in H2O
mp
172-175 °C (lit.)
solubility
water: soluble 25 mg/mL, clear, colorless
SMILES string
O=C([C@@H]([C@@H](O1)[C@H](O)[C@H](O)C1=O)O)[H]
InChI
1S/C6H8O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h1-5,8-10H/t2-,3+,4-,5+/m0/s1
InChI key
UYUXSRADSPPKRZ-SKNVOMKLSA-N
Looking for similar products? Visit Product Comparison Guide
General description
D-(+)-Glucuronic acid γ-lactone (Glucourono-γ-lactone, Glucurone or Glycurone) is a carbohydrate derivative. It converted into L-ascorbic acid in animals and human body. Its molecule contains two five-membered rings. Its crystal structure has been studied.
Application
D-(+)-Glucuronic acid γ-lactone may be used in the following studies:
- As starting ragent in the synthesis of 2,3,4,-tris(tert.-butyldimethysilyl) glucuronic acid trichloroethylester, required for the preparation of 1-O-acyl glucuronide of the anti-inflammatory drug ML-3000.
- Synthesis of optically active glucopyranoses.
- Synthesis of long-chain alkyl glucofuranosides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemical & Pharmaceutical Bulletin, 41, 1197-1197 (1993)
The crystal structure of beta-D-glucurono-gamma-lactone.
S H Kim et al.
Acta crystallographica, 22(5), 733-743 (1967-05-10)
P Florio et al.
Carbohydrate research, 328(4), 445-448 (2000-11-28)
A concise route to novel mimetics of Kdn2en, based on delta4-uronic acids, from D-glucurono-6,3-lactone is presented. Uronic acid-based mimetics in which an aliphatic ether (O-glycoside), a thioether (S-glycoside), or acetamide takes the place of the natural C-6 glycerol sidechain of
J A Horne et al.
Amino acids, 20(1), 83-89 (2001-04-20)
500 ml of a glucose based "energy" drink versus a control without the active ingredients (caffeine, taurine, glucuronolactone) were given double blind to 11 sleepy participants driving an interactive real-car driving simulator. Lane drifting and a secondary task (reaction time)
Rongchun Wang et al.
Bioscience, biotechnology, and biochemistry, 74(3), 601-605 (2010-03-09)
The degradation kinetics of glucuronic acid (GlcA) under subcritical conditions from 160 to 200 degrees C was studied in a continuous tubular reactor. The formation of glucuronolactone (GlcL) during the treatment of GlcA in subcritical water was substantiated by ESI-TOF-MS
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service