All Photos(3)
About This Item
Empirical Formula (Hill Notation):
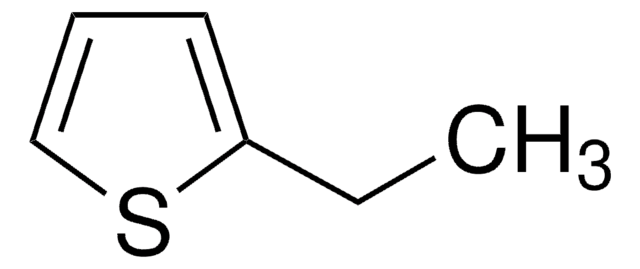
C6H4S
CAS Number:
Molecular Weight:
108.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
refractive index
n20/D 1.5800 (lit.)
bp
152-153 °C (lit.)
density
1.098 g/mL at 25 °C (lit.)
SMILES string
C#Cc1ccsc1
InChI
1S/C6H4S/c1-2-6-3-4-7-5-6/h1,3-5H
InChI key
MJHLPKWONJUCFK-UHFFFAOYSA-N
Application
3-Ethynylthiophene may be used in the synthesis of the following:
- 1-(2-bromobenzyl)-4-(thiophen-3-yl)-1H-1,2,3-triazole which is obtained by heating with 2-iodophenylethylazide in the presence of copper acetate monohydrate catalyst in N-methyl-2-pyrrolidone
- N-benzyl-1-phenyl-5-(thiophen-3-yl)-4-pentyn-2-amine via a multi-step reaction process
- [(C4H3S-3)C≡CAg]n, a polymeric compound obtained via reaction with silver nitrate in the presence of triethylamine in acetonitrile
- 4,5-bis(thiophen-3-ylethynyl)phthalonitrile via Sonogashira cross-coupling reaction with 4,5-dichlorophthalonitrile
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
115.0 °F - closed cup
Flash Point(C)
46.1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Synthesis and photophysical properties of novel unsymmetrical metal-free and metallophthalocyanines"
Ozcesmeci I, et al.
Journal of Organometallic Chemistry, 750, 125-131 (2014)
"Synthesis, analysis of spectroscopic and nonlinear optical properties of the novel compound:(S)-N-benzyl-1-phenyl-5-(thiophen-3-yl)-4-pentyn-2-amine"
Karabacak M, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 97, 556-567 (2012)
"Silver (I)- Thiophene p Interaction in the Assembly of Coordination Networks with the Supramolecular Synthons R- C? C? Ag n (R= 2-or 3-thienyl; n= 4)"
Zhao L, et al.
Organometallics, 26(18), 4439- 4448 (2007)
"An easy synthetic approach to 1, 2, 3-triazole-fused heterocycles"
Fiandanese V, et al.
Tetrahedron, 66(46), 8846-8853 (2010)
Qing Li et al.
Marine drugs, 16(4) (2018-03-31)
Chitosan is an abundant and renewable polysaccharide, which exhibits attractive bioactivities and natural properties. Improvement such as chemical modification of chitosan is often performed for its potential of providing high bioactivity and good water solubility. A new class of chitosan
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service