All Photos(1)
About This Item
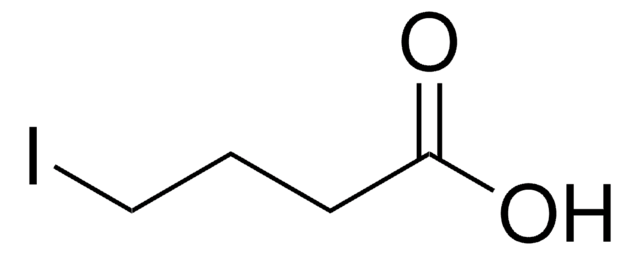
Linear Formula:
I(CH2)3CO2CH3
CAS Number:
Molecular Weight:
228.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
contains
copper as stabilizer
refractive index
n20/D 1.505 (lit.)
bp
80-83 °C/11 mmHg (lit.)
density
1.689 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
COC(=O)CCCI
InChI
1S/C5H9IO2/c1-8-5(7)3-2-4-6/h2-4H2,1H3
InChI key
NBCIIVXSBPDKOM-UHFFFAOYSA-N
General description
Methyl 4-iodobutyrate can be synthesized from methyl-4-chlorobutyrate and sodium iodide.
Application
Methyl 4-iodobutyrate may be used as reagent in the synthesis of 4-[formyl-5-(methoxymethyl)-1H-pyrrol-1-yl] butanoic acid. It has been used in the synthesis of a range of stable S-adenosylmethionine (SAM) mimetics. Their ability to promote the binding of the E. coli methionine repressor (MetJ) to its operator DNA has been investigated.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Catherine Joce et al.
Organic & biomolecular chemistry, 7(4), 635-638 (2009-02-06)
The efficient synthesis of a range of stable SAM mimetics, and their ability to promote the binding of the E. coli methionine repressor (MetJ) to its operator DNA, is described.
Young-Won Chin et al.
Bioorganic & medicinal chemistry letters, 13(1), 79-81 (2002-12-07)
As a part of our search for hepatoprotective compounds from Lycium chinense fruits, three new pyrrole derivatives (1-3) were isolated. These compounds and a related synthetic methylated compound (4) were evaluated for their biological activity and structure-activity relationship, and compounds
IgE-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone.
Himly M, et al.
The Journal of Allergy and Clinical Immunology, 111(4), 882-888 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service