All Photos(1)
About This Item
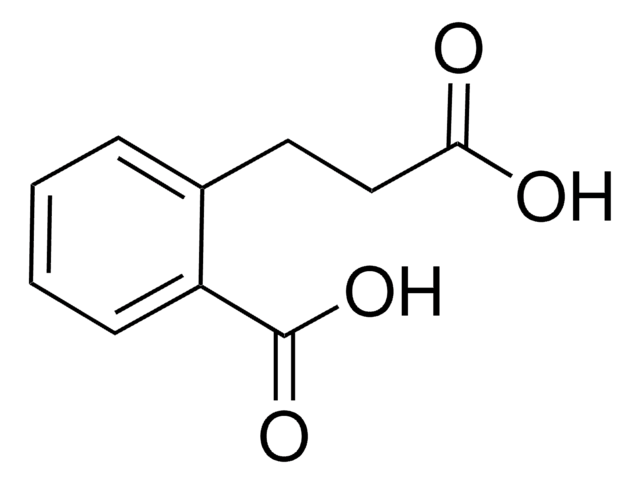
Linear Formula:
HOC6H4CH2CH2CO2H
CAS Number:
Molecular Weight:
166.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
86-89 °C (lit.)
SMILES string
OC(=O)CCc1ccccc1O
InChI
1S/C9H10O3/c10-8-4-2-1-3-7(8)5-6-9(11)12/h1-4,10H,5-6H2,(H,11,12)
InChI key
CJBDUOMQLFKVQC-UHFFFAOYSA-N
General description
3-(2-Hydroxyphenyl) propionic acid is a phenyl propionic acid derivative. It was found to be one of the constituents of Justicia pectoralis Jacq. extract which was analyzed by gas chromatography/mass spectrometry (GC/MS). The antiulcerogenic effect of 3-(2-hydroxyphenyl) propionic acid in the prevention of serotonin-induced ulcerogenesis has been studied in rats. It has been reported to be one of the major microbial metabolite of both (+)-catechin and (-)-epicatechin by human faecal microbiota. Crystal structure study reveals that 3-(2-hydroxyphenyl)propionic acid crystals are monoclinic with space group P21/c.
Application
3-(2-Hydroxyphenyl)propionic acid is suitable as a growth substrate for various strains of E. coli and as a standard in the study of microbial metabolism of catechin stereoisomers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Microbial metabolism of catechin stereoisomers by human faecal microbiota: comparison of targeted analysis and a non-targeted metabolomics method.
Aura AM, et al.
Phytochemistry Letters, 1(1), 18-22 (2008)
Structure of 3-(2-hydroxyphenyl) propionic acid.
Begum NS, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1076-1078 (1992)
R Burlingame et al.
Journal of bacteriology, 155(1), 113-121 (1983-07-01)
A number of laboratory strains and clinical isolates of Escherichia coli utilized several aromatic acids as sole sources of carbon for growth. E. coli K-12 used separate reactions to convert 3-phenylpropionic and 3-(3-hydroxyphenyl)propionic acids into 3-(2,3-dihydroxyphenyl)propionic acid which, after meta-fission
S Tanaka et al.
Planta medica, 55(3), 245-248 (1989-06-01)
Two active compounds that prevent serotonin-induced ulcerogenesis in rats were isolated from Chinese cinnamon (the stem bark of Cinnamomum cassia) and identified as 3-(2-hydroxyphenyl)-propanoic acid and its O-glucoside. The former compound, administered orally or parenterally to rats at a remarkably
J X de Vries et al.
Biomedical & environmental mass spectrometry, 15(8), 413-417 (1988-04-15)
The analysis of extracts from the South American plant Justicia pectoralis Jacq. permitted the identification, among other compounds, of coumarin, dihydrocoumarin, umbelliferone and 3-(2-hydroxyphenyl)propionic acid by gas chromatography/mass spectrometry (GC/MS); the acids and phenolic compounds were derivatized with diazomethane. GC/MS
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service